No products in the cart.
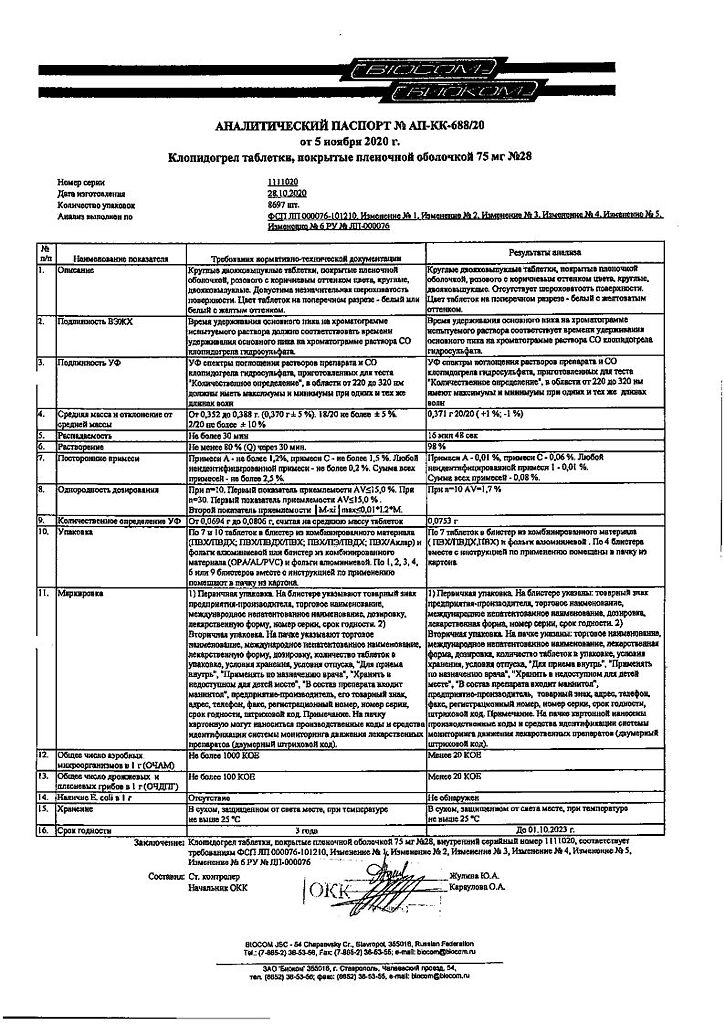
Clopidogrel, 75 mg 28 pcs
€14.75 €12.29
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group
Antiplatelet drug
ATC code
B01AC04
Pharmacodynamics:
Clopidogrel is a prodrug one of whose active metabolites is a platelet aggregation inhibitor.
The active metabolite of clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet receptor P2Y12 and the subsequent ADP-dependent activation of glycoprotein complex IIb/IIIa, thereby suppressing platelet aggregation. Reduces platelet aggregation caused by other agonists (other than ADP) without affecting phosphodiesterase activity.
Due to the irreversibility of the binding, the exposed platelets are damaged for the rest of their life (approximately 7-10 days) and the restoration of normal platelet function occurs at a rate corresponding to the platelet cycle. Platelet aggregation caused by agonists other than ADP is also inhibited by blocking the enhanced platelet activation that occurs under the influence of the released ADP.
As the active metabolite of clopidogrel is formed with the CYP2C19 isoenzyme, some of which are polymorphic or inhibited by other compound medications, platelet suppression is not sufficient in all patients.
When clopidogrel is taken daily at a dose of 75 mg from the first day there is a significant suppression of ADP-induced platelet aggregation that gradually increases over 3-7 days and then an equilibrium state is reached (reaches a constant level). In the equilibrium state platelet aggregation is suppressed by an average of 40-60%. After stopping clopidogrel administration, platelet aggregation and bleeding time return to the initial level on average within 5 days.
Clopidogrel is able to prevent the development of atherothrombosis in any localization of atherosclerotic vascular lesions, particularly in lesions of the cerebral coronary or peripheral arteries.
In patients with recent myocardial infarction ischemic stroke and/or diagnosed peripheral arterial occlusive disease, administration of clopidogrel at a dose of 75 mg daily significantly reduces the risk of vascular complications (myocardial infarction stroke cardiovascular mortality).
In acute coronary syndrome without ST-segment elevation on ECG (unstable angina pectoris myocardialis infarction) administration of clopidogrel (loading dose of 300 mg once and then 75 mg/day) in combination with acetylsalicylic acid 75-325 mg/day and other standard therapy significantly and independently of other therapy reduces risk of vascular complications.
. In ST-segment elevation myocardial infarction on ECG, administration of clopidogrel (loading dose of 300 mg once during the first 12 hours of illness then 75 mg/day) in combination with acetylsalicylic acid (loading dose of 150-325 mg then 75-162 mg/day) fibrinolytic therapy and, when indicated, heparin reduces the incidence of infarct-related coronary artery occlusion (according to coronary angiography at discharge from hospital) of repeated myocardial infarction and fatal outcomes.
In patients for whom coronary angiography was not performed at discharge, clopidogrel administration according to this regimen reduces the incidence of fatal outcomes and recurrent myocardial infarction until day 8 of illness or until discharge from the hospital.
In general, in myocardial infarction, regardless of ECG changes (ST-segment elevation ST depression or first-ever complete left bundle branch block), clopidogrel at a dose of 75 mg/day in combination with acetylsalicylic acid 162 mg daily leads to a decrease in overall mortality and cumulative incidence of recurrent myocardial infarction ischemic stroke and fatal outcomes.
. Results from a clinical trial showed that in patients with atrial fibrillation who had at least one risk factor for vascular complications but were unable to take indirect anticoagulants, clopidogrel combined with acetylsalicylic acid (compared with use of acetylsalicylic acid alone) reduced the cumulative incidence of myocardial infarction systemic thromboembolism outside the central nervous system or vascular death by a greater reduction in stroke risk.
Pharmacokinetics:
Intake and distribution: Clopidogrel at a regular oral dose of 75 mg/day is rapidly absorbed.
The maximum concentration (Cmax) of unchanged clopidogrel after a single oral dose of 75 mg is approximately 22-25 ng/mL; the time to maximum concentration (TCmax) of clopidogrel is approximately 45 minutes. Based on the data on excretion of clopidogrel metabolite by the kidneys, the absorption of clopidogrel is at least 50%.
In vitro clopidogrel and its main circulating inactive metabolite are reversibly bound to plasma proteins (98% and 94%, respectively) this bond is unsaturated in a wide range of concentrations.
Metabolism: being a prodrug clopidogrel is extensively metabolized in the liver. In vivo and in vitro clopidogrel is metabolized in two ways: the first – through enzymes and subsequent hydrolysis with the formation of inactive carboxylic acid derivative (85% of circulating metabolites) the second way – through cytochrome P450 isoenzyme system. Initially, clopidogrel is metabolized to 2-oxo-clopidogrel, which is an intermediate metabolite. Subsequent metabolism of 2-oxo-clopidogrel leads to the formation of the active metabolite of clopidogrel – thiol derivative of clopidogrel. In vitro, this metabolic pathway occurs via the CYP3A4 CYP2C19 CYP1A2 and CYP2B6 isoenzymes.
The active thiol metabolite can only be isolated in in vitro studies; under in vivo conditions, it binds rapidly and irreversibly to the P2Y12 receptor of platelets, thereby inhibiting platelet aggregation.
The cmax of active clopidogrel metabolite after administration of its loading dose of 300 mg is 2 times higher than cmax after 4 days of maintenance dose of clopidogrel 75 mg. Moreover, when clopidogrel 300 mg is taken, the Cmax is reached within approximately 30-60 minutes.
After repeated oral administration of clopidogrel at a dose of 75 mg per day, the Cmax of the main inactive metabolite is approximately 3 mg/L. The Cmax of the inactive metabolite is reached after 1 hour.
Elimation: within 120 hours after human oral administration of 14C-labeled clopidogrel about 50% of the dose is excreted by the kidneys and approximately 46% – through the intestine.
After a single oral dose of 75 mg, the elimination half-life (T1/2) is approximately 6 hours. After a single dose and repeated doses, the T1/2 of its main metabolite is 8 hours.
Pharmacogenetics
The active metabolite as well as the intermediate metabolite 2-oxo-clopidogrel are formed using CYP2C19 isoenzyme. Pharmacokinetics and anti-aggregant effect of the active metabolite clopidogrel in platelet aggregation studiesin vivo vary depending on the genotype of CYP2C19 isoenzyme. Allele of CYP2C19*1 gene corresponds to a fully functional metabolism while alleles of CYP2C19*2 and CYP2C19*3 genes cause decreased metabolism in the majority of representatives of Caucasoid (85%) and Mongoloid race (99%). Other alleles associated with absence or reduced metabolism are less common and include, but are not limited to, CYP2C19*4 *5 *6 *7 and *8 alleles. Patients with low CYP2C19 isoenzyme activity should have the two loss-of-function gene alleles listed above. The published frequencies of phenotypes of patients with low CYP2C19 isoenzyme activity are 2% in Caucasoid races, 4% in non-Hispanic races, and 14% in Chinese.
Tests are available to determine a patient’s CYP2C19 genotype these tests can be used as an adjunctive tool in determining treatment tactics.
In a cross-sectional study involving 40 healthy volunteers (10 volunteers each with very fast extensive intermediate and delayed metabolism), the pharmacokinetics and antiaggregant effects of clopidogrel were studied when administered according to two regimens: 1) 300 mg once followed by a dose of 75 mg per day for 5 days and 2) 600 mg once followed by a dose of 150 mg per day for 5 days (until equilibrium was reached). No significant differences in active metabolite exposure and mean platelet aggregation inhibition (IAT) were detected between the groups of volunteers with very rapid extensive and intermediate metabolism. Volunteers with reduced metabolic rate had 63-71% lower exposure to active metabolite compared to volunteers extensively metabolizing clopidogrel.
When the drug was administered at 300 mg once / 75 mg daily, the platelet response (when stimulated by 5 μm ADP) was reduced in volunteers with delayed clopidogrel metabolism: IAT was 24% (after 24 hours) and 37% (Day 5). In comparison, volunteers with extensive metabolism had an IAT of 39% (after 24 hours) and 60% (Day 5).
When clopidogrel was administered at 600 mg once/150 mg daily in volunteers with reduced clopidogrel metabolic rate, the effects of the drug were more pronounced than when the drug was administered at 300 mg once/75 mg daily doses: IAT was 32% (after 24 hours) and 61% (Day 5), which was comparable to IAT in groups of volunteers with other clopidogrel metabolic rates receiving the drug at the 300 mg/75 mg regimen. A suitable dosing regimen for a population of patients with reduced clopidogrel metabolic rate has not been established in clinical trials.
A similar meta-analysis of six studies that included data from 335 healthy volunteers who received clopidogrel and were at equilibrium concentration reached showed that intermediate metabolizers had a 28% reduction in exposure to the active metabolite and weak metabolizers had a 72% reduction. Compared to intensive metabolizers, inhibition of platelet aggregation was reduced by 59% and 214% in intermediate and weak metabolizers, respectively.
The effect of SUR2C19 genotype on clinical outcomes in patients treated with clopidogrel has not been evaluated in prospective randomized controlled trials. Existing data from retrospective analyses of clinical trials for which patient genotyping results are available do not have sufficient power to assess differences in clinical outcomes in weak clopidogrel metabolizers compared with intensive and intermediate metabolizers.
Particular patient groups
Elderly persons
There were no differences in platelet aggregation and bleeding time in elderly volunteers (over 75 years) when compared with younger volunteers. No dose adjustment of clopidogrel in the elderly is required.
Age under 18 years
The pharmacokinetics of clopidogrel in children has not been studied.
Kidney function impairment
. After repeated administration of clopidogrel at a dose of 75 mg/day in patients with severe chronic renal failure (creatinine clearance (CK) 5-15 ml/min), inhibition of ADP-induced platelet aggregation was 25% lower than in healthy volunteers, but no prolongation of bleeding time was observed.
Hepatic impairment
After administration of clopidogrel at a dose of 75 mg daily for 10 days in patients with severe liver injury, inhibition of ADP-induced platelet aggregation did not differ from that in healthy volunteers. The mean bleeding time in patients with severe liver injury and in healthy volunteers was comparable.
Raciality
The prevalence of CYP2C19 isoenzyme gene alleles responsible for intermediate or reduced metabolism differs among racial groups. The available literature is insufficient to evaluate the value of CYP2C19 isoenzyme genotyping for predicting ischemic complications.
Indications
Indications
Prevention of atherothrombotic disorders in adult patients:
– after a myocardial infarction (with a duration from several days to less than 35 days) ischemic stroke (with a duration from 7 days to less than 6 months) or with diagnosed occlusive disease of the peripheral arteries;
– with acute coronary syndrome:
without ST segment elevation (unstable angina or myocardial infarction without Q wave), incl. in patients who underwent stenting during percutaneous coronary intervention (in combination with acetylsalicylic acid);
with ST segment elevation (acute myocardial infarction with ST segment elevation) with drug treatment and the possibility of thrombolysis (in combination with acetylsalicylic acid);
Prevention of atherothrombotic and thromboembolic complications including stroke in atrial fibrillation (atrial fibrillation)
– in adult patients with atrial fibrillation (atrial fibrillation) who have at least one risk factor for the development of vascular complications, they cannot take indirect anticoagulants and have a low risk of bleeding (in combination with acetylsalicylic acid).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Antiplatelet agent
ATX code
B01AC04
Pharmacodynamics:
Clopidogrel is a prodrug whose active metabolite is an inhibitor of platelet aggregation.
The active metabolite of clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet receptor P2Y12 and subsequent ADP-mediated activation of the glycoprotein IIb/IIIa complex, thereby inhibiting platelet aggregation. Reduces platelet aggregation caused by other agonists (other than ADP) does not affect phosphodiesterase activity.
Due to the irreversibility of binding, exposed platelets are damaged for the remainder of their life (approximately 7-10 days) and restoration of normal platelet function occurs at a rate consistent with the platelet cycle. Platelet aggregation caused by agonists other than ADP is also inhibited by blocking the enhanced platelet activation that occurs under the influence of released ADP.
Since the active metabolite of clopidogrel is formed with the participation of the CYP2C19 isoenzyme, some of which are polymorphic or inhibited by other drugs, platelet suppression is not sufficient in all patients.
When taking clopidogrel daily at a dose of 75 mg, from the first day there is a significant suppression of ADP-induced platelet aggregation, which gradually increases over 3-7 days and then reaches an equilibrium state (reaches a constant level). At steady state, platelet aggregation is suppressed by an average of 40-60%. After discontinuation of clopidogrel, platelet aggregation and bleeding time returned to baseline levels within an average of 5 days.
Clopidogrel is able to prevent the development of atherothrombosis in any localization of atherosclerotic vascular lesions, in particular in lesions of the cerebral coronary or peripheral arteries.
In patients with recent myocardial infarction, ischemic stroke and/or diagnosed occlusive peripheral artery disease, taking clopidogrel at a dose of 75 mg per day significantly reduces the risk of developing vascular complications (myocardial infarction, stroke, cardiovascular mortality).
In acute coronary syndrome without ST segment elevation on the ECG (unstable angina, myocardial infarction), taking clopidogrel (loading dose of 300 mg once, then 75 mg/day) in combination with acetylsalicylic acid at a dose of 75-325 mg/day and other standard therapy reliably and independently of other types of treatment reduces the risk of developing vascular complications.
In myocardial infarction with ST segment elevation on the ECG, taking clopidogrel (loading dose 300 mg once during the first 12 hours of the disease, then 75 mg/day) in combination with acetylsalicylic acid (loading dose 150-325 mg then 75-162 mg/day) fibrinolytic therapy and, if indicated, heparin reduces the frequency of occlusion of the infarct-related coronary artery (according to coronary angiography at discharge from the hospital), recurrent myocardial infarction and deaths.
In patients who did not undergo coronary angiography upon discharge, taking clopidogrel according to the specified regimen reduces the incidence of deaths and recurrent myocardial infarction until the 8th day of illness or until discharge from the hospital.
In general, in case of myocardial infarction, regardless of ECG changes (ST segment elevation, ST segment depression or new complete block of the left bundle branch), taking clopidogrel at a dose of 75 mg/day in combination with acetylsalicylic acid 162 mg per day leads to a decrease in overall mortality and the total incidence of recurrent myocardial infarction, ischemic stroke and deaths.
The results of a clinical trial showed that in patients with atrial fibrillation who had at least one risk factor for the development of vascular complications but were unable to take indirect anticoagulants, clopidogrel in combination with acetylsalicylic acid (compared with acetylsalicylic acid alone) reduced the total incidence of stroke, myocardial infarction, systemic thromboembolism outside the central nervous system, or vascular death to a greater extent due to reducing the risk of stroke.
Pharmacokinetics:
Absorption and distribution: with regular oral administration of clopidogrel at a dose of 75 mg/day, it is rapidly absorbed.
The maximum concentration (Cmax) of unchanged clopidogrel after a single oral dose of 75 mg is approximately 22-25 ng/ml; the time to reach maximum concentration (TCmax) of clopidogrel is approximately 45 minutes. Based on data on the excretion of the clopidogrel metabolite by the kidneys, the absorption of clopidogrel is at least 50%.
In vitro, clopidogrel and its main inactive metabolite circulating in the blood are reversibly bound to plasma proteins (98% and 94%, respectively); this bond is unsaturated over a wide range of concentrations.
Metabolism: being a prodrug, clopidogrel is extensively metabolized in the liver. In vivo and in vitro, clopidogrel is metabolized in two ways: the first – through enzymes and subsequent hydrolysis with the formation of an inactive carboxylic acid derivative (85% of circulating metabolites); the second way – through the cytochrome P450 isoenzyme system. Initially, clopidogrel is metabolized to 2-oxo-clopidogrel, which is an intermediate metabolite. Subsequent metabolism of 2-oxo-clopidogrel leads to the formation of the active metabolite of clopidogrel – the thiol derivative of clopidogrel. In vitro, this metabolic pathway occurs through the isoenzymes CYP3A4 CYP2C19 CYP1A2 and CYP2B6.
The active thiol metabolite can only be isolated in in vitro studies; under in vivo conditions, it rapidly and irreversibly binds to the platelet P2Y12 receptor, thereby inhibiting platelet aggregation.
The Cmax of the active metabolite of clopidogrel after taking a loading dose of 300 mg is 2 times higher than the Cmax after 4 days of taking a maintenance dose of 75 mg clopidogrel. In this case, when taking 300 mg of clopidogrel, Cmax is achieved within approximately 30-60 minutes.
After repeated oral administration of clopidogrel at a dose of 75 mg per day, the Cmax of the main inactive metabolite is about 3 mg/l. The Cmax of the inactive metabolite is reached after 1 hour.
Elimination: Within 120 hours of human ingestion of 14C-labeled clopidogrel, approximately 50% of the dose is excreted by the kidneys and approximately 46% by the intestines.
After a single oral dose of 75 mg, the half-life (T1/2) is approximately 6 hours. After a single dose and repeated doses, T1/2 of its main metabolite is 8 hours.
Pharmacogenetics
With the help of the CYP2C19 isoenzyme, both the active metabolite and the intermediate metabolite – 2-oxo-clopidogrel – are formed. The pharmacokinetics and antiplatelet effect of the active metabolite of clopidogrel in in vivo platelet aggregation studies vary depending on the genotype of the CYP2C19 isoenzyme. The allele of the CYP2C19*1 gene corresponds to a fully functional metabolism, while the alleles of the CYP2C19*2 and CYP2C19*3 genes are the cause of decreased metabolism in the majority of representatives of the Caucasian (85%) and Mongoloid races (99%). Other alleles associated with absent or decreased metabolism are less common and include, but are not limited to, alleles of the CYP2C19*4 *5 *6 *7 and *8 genes. Patients with low CYP2C19 activity must have the two loss-of-function alleles listed above. The published frequency of occurrence of phenotypes in patients with low activity of the CYP2C19 isoenzyme is 2% in Caucasians, 4% in Blacks, and 14% in Chinese.
There are tests to determine a patient’s CYP2C19 genotype; these tests can be used as an auxiliary tool in determining treatment tactics.
In a crossover study involving 40 healthy volunteers (10 volunteers each with very rapid extensive intermediary and slow metabolizers), the pharmacokinetics and antiplatelet effect of clopidogrel were studied when administered according to two regimens: 1) 300 mg once followed by a dose of 75 mg per day for 5 days and 2) 600 mg once followed by a dose of 150 mg every day. day for 5 days (until equilibrium is achieved). There were no significant differences in active metabolite exposure and mean platelet aggregation inhibition (PAI) levels between the very rapid extensive metabolizer and intermediate metabolizer groups. In volunteers with a reduced metabolic rate, exposure to the active metabolite was 63-71% lower compared to volunteers who extensively metabolized clopidogrel.
When using the drug according to the regimen of 300 mg once / 75 mg per day, the platelet response (when stimulated with 5 μM ADP) in volunteers with slow metabolism of clopidogrel was reduced: IAT was 24% (after 24 hours) and 37% (Day 5). For comparison, in volunteers with extensive metabolism, the IAT was 39% (at 24 hours) and 60% (Day 5).
When using clopidogrel in a regimen of 600 mg once / 150 mg per day in volunteers with a reduced rate of metabolism of clopidogrel, the effect of the drug was more pronounced than when using the drug in doses of 300 mg once / 75 mg per day: the IAT was 32% (after 24 hours) and 61% (Day 5), which is comparable to the IAT values in groups of volunteers with a different rate of metabolism of clopidogrel receiving the drug according to the 300 mg/75 mg regimen. The appropriate dosing regimen for the patient population with a reduced rate of metabolism of clopidogrel has not been established in clinical studies.
Similarly, a meta-analysis of six studies that included data from 335 healthy volunteers treated with clopidogrel at steady state found that intermediate metabolizers reduced exposure to the active metabolite by 28% and poor metabolizers reduced exposure to the active metabolite by 72%. Compared with extensive metabolizers, inhibition of platelet aggregation in intermediate and poor metabolizers was reduced by 59% and 214%, respectively.
The effect of the CYP2C19 genotype on clinical outcomes in patients receiving clopidogrel has not been assessed in prospective randomized controlled trials. Existing data from retrospective analyzes of clinical trials for which patient genotyping results are available are not sufficiently powered to assess differences in clinical outcomes in poor metabolizers of clopidogrel compared with extensive and intermediate metabolizers.
Special patient groups
Elderly people
In elderly volunteers (over 75 years of age), when compared with young volunteers, there were no differences in platelet aggregation and bleeding time. No dose adjustment of clopidogrel is required in elderly patients.
Age under 18
The pharmacokinetics of clopidogrel in children has not been studied.
Renal dysfunction
After repeated doses of clopidogrel at a dose of 75 mg/day in patients with severe chronic renal failure (creatinine clearance (CR) 5-15 ml/min), the inhibition of ADP-induced platelet aggregation was 25% lower than in healthy volunteers; however, no prolongation of bleeding time was observed.
Liver dysfunction
After administration of clopidogrel at a dose of 75 mg per day for 10 days in patients with severe liver damage, the inhibition of ADP-induced platelet aggregation did not differ from that in healthy volunteers. The mean bleeding time was comparable in patients with severe liver damage and in healthy volunteers.
Race
The prevalence of alleles of the CYP2C19 isoenzyme genes responsible for intermediate or reduced metabolism differs among representatives of different racial groups. The available literature data are insufficient to assess the value of genotyping the CYP2C19 isoenzyme for predicting the development of ischemic complications.
Special instructions
Special instructions
It is necessary to clarify whether patients have a history of hypersensitivity to other thienopyridine derivatives (ticlopidine prasugrel) because Cases of cross-allergic reactions between thienopyridines have been described. Patients who have previously experienced hypersensitivity to other thienopyridines require careful monitoring throughout the entire period of therapy to identify signs of hypersensitivity to clopidogrel.
During the treatment period, it is necessary to monitor the indicators of the hemostatic system (activated partial thromboplastin time (APTT), platelet count, tests of platelet functional activity); regularly examine the functional activity of the liver.
Clopidogrel should be used with caution in patients at risk of significant bleeding during trauma and surgery, in patients with injuries prone to bleeding (especially gastrointestinal and intraocular), as well as in patients receiving acetylsalicylic acid, non-steroidal anti-inflammatory drugs (including COX-2 inhibitors), heparin or glycoprotein IIb/IIIa inhibitors, SSRIs and thrombolytic drugs. Patients should be closely monitored for any signs of bleeding, including occult bleeding, especially during the first weeks of drug use and/or after invasive cardiac procedures or surgery.
The simultaneous use of clopidogrel and warfarin should be done with extreme caution as it may increase bleeding.
In the case of surgical interventions, if the antiplatelet effect is undesirable, the course of treatment should be discontinued 7 days before surgery.
Patients should be warned that since stopping bleeding occurring during the use of clopidogrel (in combination with or without acetylsalicylic acid) requires more time, they should inform the doctor about each case of bleeding. Patients should also inform their doctor about taking the drug if they are undergoing surgery.
After taking clopidogrel, thrombotic thrombocytopenic purpura (TTP) was detected very rarely, sometimes after short-term use. This condition is characterized by thrombocytopenia and microangiopathic hemolytic anemia in combination with neurological signs of renal dysfunction or fever. TTP is a potentially fatal condition that requires immediate treatment, including plasmapheresis.
Taking clopidogrel cannot be recommended in the acute period (less than 7 days) of ischemic stroke due to the lack of data on the effectiveness and safety of its use.
In patients with recent ischemic stroke or transient ischemic attack and a high risk of recurrent atherothrombotic events, combination therapy with clopidogrel and acetylsalicylic acid has not been shown to be superior to clopidogrel monotherapy but may increase the risk of major bleeding.
Acquired hemophilia
Cases of acquired hemophilia have been reported when taking clopidogrel. With a confirmed isolated increase in activated partial thromboplastin time (APTT), accompanied or not accompanied by the development of bleeding, the possibility of developing acquired hemophilia should be suspected. Patients with a confirmed diagnosis of acquired hemophilia should be observed and treated by specialists in this disease. Clopidogrel should be discontinued.
Cross allergic and/or hematological reactions between thienopyridines
It is necessary to clarify whether patients have a history of allergic and/or hematological reactions to other thienopyridine derivatives (such as ticlopidine and prasugrel) because Cases of cross allergic and/or hematological reactions between thienopyridines have been described. Thienopyridines may cause moderate to severe allergic reactions (rash, angioedema) or hematological reactions (thrombocytopenia, neutropenia).
Patients who have previously experienced allergic and/or hematological reactions to one of the thienopyridine derivatives may have an increased risk of developing such reactions when taking another thienopyridine drug. Such patients require careful monitoring throughout the entire period of therapy to identify signs of hypersensitivity to clopidogrel.
Experience with the use of clopidogrel in patients with impaired renal function is limited; therefore, clopidogrel should be prescribed to such patients with caution.
In case of severe liver dysfunction, one should remember the risk of developing hemorrhagic diathesis. Experience with the drug in patients with moderate liver dysfunction is limited; therefore, clopidogrel should be prescribed to these patients with caution.
Since Clopidogrel contains lactose as an excipient, it should not be taken by patients with rare hereditary lactose intolerance, lactase deficiency and glucose-galactose malabsorption syndrome (see section “Contraindications”).
Impact on the ability to drive vehicles. Wed and fur.:
Clopidogrel may cause side effects from the nervous system (headache, dizziness, systemic dizziness, confusion, hallucinations) that may affect the ability to drive vehicles and engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions. However, in most cases, clopidogrel does not have a significant effect on the ability to drive vehicles and operate machinery.
Active ingredient
Active ingredient
Clopidogrel
Composition
Composition
For one tablet:
Active substance: clopidogrel hydrosulfate – 97.88 mg, in terms of clopidogrel – 75.00 mg.
Excipients (core): lactose – 70.00 mg, microcrystalline cellulose – 17.00 mg, croscarmellose sodium – 5.52 mg, colloidal silicon dioxide – 1.80 mg, magnesium stearate – 1.80 mg.
Excipients (shell): hypromellose – 3.30 mg, macrogol-4000 – 0.90 mg, red iron oxide dye – 0.06 mg, titanium dioxide – 1.74 mg.
Pregnancy
Pregnancy
The use of the drug Clopidogrel is not recommended during pregnancy due to the lack of clinical data on its use in pregnant women, although animal studies have not revealed any direct or indirect undesirable effects on pregnancy, embryonic development, childbirth and postnatal development.
Studies in rats have shown that clopidogrel and/or its metabolites are excreted in the milk of lactating rats. There are no data on the excretion of clopidogrel into breast milk. During therapy with Clopidogrel, it is recommended to stop breastfeeding.
Fertility
Animal studies have not revealed any adverse effects of clopidogrel on fertility.
Contraindications
Contraindications
– Hypersensitivity to clopidogrel or any of the excipients included in the drug.
– Severe liver failure.
– Active bleeding (including bleeding from a peptic ulcer or intracranial hemorrhage).
– Pregnancy and breastfeeding period.
– Age up to 18 years (efficacy and safety of use have not been established).
– Rare hereditary lactose intolerance, lactase deficiency and glucose-galactose malabsorption syndrome (due to the presence of lactose in the drug).
With caution:
– Moderate liver failure (7-9 points on the Child-Pugh scale) (with a possible predisposition to bleeding) – experience with clinical use is limited;
– chronic renal failure (CRF) of mild to moderate severity (creatinine clearance 60-30 ml/min) – experience of clinical use is limited;
– diseases in which there is a predisposition to the development of bleeding (especially gastrointestinal or intraocular), incl. in case of trauma and surgical interventions;
– simultaneous use of non-steroidal anti-inflammatory drugs (NSAIDs) (including cyclooxygenase-2 (COX-2) inhibitors);
– simultaneous use of oral anticoagulants, heparin, inhibitors of glycoprotein IIb/IIIa receptors, selective serotonin reuptake inhibitors (SSRIs) and thrombolytic drugs;
– hereditary decrease in the activity of the CYP2C19 isoenzyme (since when clopidogrel is used in recommended doses, less of the active metabolite of clopidogrel is formed and its antiplatelet effect is less pronounced, and therefore, when taking the usually recommended doses of clopidogrel in acute coronary syndrome or percutaneous coronary intervention, a higher incidence of cardiovascular complications is possible than in patients with normal isoenzyme activity CYP2C19);
– if you have a history of hypersensitivity reactions to other thienopyridines (such as ticlopidine prasugrel) due to the possibility of cross-hypersensitivity reactions (see section “Special instructions”).
Side Effects
Side Effects
The frequency of adverse reactions is determined as follows: very often – more than 1/10 often – more than 1/100 and less than 1/10 infrequently – more than 1/1000 and less than 1/100 rarely – more than 1/10,000 and less than 1/1000 very rarely – less than 1/10,000 including individual messages unknown frequency (it is impossible to determine the frequency of occurrence of an adverse reaction from the available data). Within each system-organ frequency class, undesirable effects are presented in order of decreasing severity.
From the hematopoietic organs: infrequently – thrombocytopenia, leukopenia, eosinophilia; rarely – neutropenia, incl. severe neutropenia; very rarely – thrombotic thrombocytopenic purpura aplastic anemia pancytopenia agranulocytosis severe thrombocytopenia granulocytopenia anemia; frequency unknown – acquired hemophilia A.
From the immune system: unknown frequency – anaphylactoid reactions, serum sickness, cross-allergic and hematological reactions with other thienopyridines (such as ticlopidine prasugrel)
From the blood coagulation system: often – hematoma, nosebleeds, gastrointestinal bleeding, bruises, bleeding at the puncture site; sometimes – intracranial bleeding, hemorrhage in the eye (conjunctival ocular retinal) prolongation of bleeding time; rarely – retroperitoneal bleeding; very rarely – severe bleeding from the surgical wound, bleeding from the respiratory system (hemoptysis, pulmonary hemorrhage), hemarthrosis.
From the nervous system: infrequently – intracranial hemorrhage, incl. fatal headache, paresthesia, dizziness; very rarely – taste disturbances.
From the mental side: very rarely – confusion, hallucinations.
From the organ of vision: infrequently – ocular hemorrhages (conjunctival in the tissue and retina of the eye).
On the part of the hearing organ: rarely – vertigo.
From the cardiovascular system: often – hematoma; very rarely – severe bleeding from the surgical wound; vasculitis; decreased blood pressure.
From the respiratory system: often – nosebleeds; very rarely – bronchospasm, interstitial pneumonitis, pulmonary hemorrhage, hemoptysis; eosinophilic pneumonia.
From the digestive system: very often – gastrointestinal bleeding, diarrhea, abdominal pain, dyspepsia; uncommon – gastric and duodenal ulcers, gastritis, vomiting, nausea, constipation, flatulence; rarely – retroperitoneal bleeding; very rarely – gastrointestinal bleeding and retroperitoneal hemorrhage with fatal outcome pancreatitis colitis (including ulcerative colitis or lymphocytic colitis) stomatitis.
From the liver and biliary tract: very rarely – hepatitis (non-infectious), acute liver failure, hepatitis, abnormal liver function indicators.
From the skin: often – subcutaneous hemorrhages; uncommon – skin rash, itchy skin, purpura; very rare / unknown frequency – bullous dermatitis (toxic epidermal necrolysis Stevens-Johnson syndrome erythema multiforme) angioedema drug hypersensitivity syndrome drug rash with eosinophilia and systemic manifestations (DRESS syndrome) erythematous or exfoliative rash urticaria eczema lichen planus
From the musculoskeletal system: very rarely – musculoskeletal hemorrhages (hemarthrosis), arthritis, arthralgia (joint pain), myalgia.
From the genitourinary system: infrequently – hematuria; very rarely – glomerulopathy (including glomerulonephritis) hypercreatininemia.
Laboratory indicators: infrequently – prolongation of bleeding time, decrease in the number of platelet neutrophils in the peripheral blood, disturbances in liver function tests, increase in the concentration of creatinine in the blood plasma.
General disorders and disorders at the injection site: often – bleeding from the site of vascular puncture; very rarely – increased body temperature.
Interaction
Interaction
Concomitant use of clopidogrel and oral anticoagulants may increase the intensity of bleeding and therefore the use of this combination is not recommended. Clopidogrel at a dose of 75 mg per day does not affect the pharmacokinetics of warfarin and does not change the International Normalized Ratio (INR) value in patients taking warfarin for a long time. However, concomitant use of warfarin with clopidogrel may increase the risk of bleeding due to the independent effects of these drugs on hemostasis.
Prescribing glycoprotein IIbIIIa receptor inhibitors together with clopidogrel requires caution, especially in patients with an increased risk of bleeding (in cases of trauma and surgery or other pathological conditions).
Acetylsalicylic acid (ASA) does not affect the clopidogrel-induced suppression of ADP-induced platelet aggregation, but clopidogrel potentiates the effect of ASA on collagen-induced platelet aggregation. However, simultaneous administration of 500 mg ASA 2 times a day did not have a significant effect on the increase in bleeding time caused by taking clopidogrel. There may be a pharmacodynamic interaction between clopidogrel and ASA, which leads to an increased risk of bleeding. Therefore, caution should be exercised when using them simultaneously, although in clinical studies patients received combination therapy with clopidogrel and ASA for one year.
When used simultaneously with heparin, according to a clinical study conducted on healthy volunteers, clopidogrel did not require a change in the dose of heparin and the anticoagulant effect of heparin did not change.
Concomitant use of heparin did not change the inhibitory effect of clopidogrel on platelet aggregation. There may be a pharmacodynamic interaction between clopidogrel and heparin, which may increase the risk of bleeding, so the use of this combination requires caution.
The safety of concomitant use of clopidogrel, fibrin-specific or non-specific thrombolytics and heparin has been studied in patients with acute myocardial infarction. The incidence of clinically significant bleeding was similar to that observed in the case of combined use of thrombolytics and heparin with ASA.
Prescribing NSAIDs (including COX-2 inhibitors) together with clopidogrel requires caution. In a clinical study conducted in healthy volunteers, coadministration of clopidogrel and naproxen increased occult gastrointestinal bleeding. However, due to the lack of studies on the interaction of clopidogrel with other NSAIDs, it is currently unknown whether there is an increased risk of gastrointestinal bleeding when taking clopidogrel together with other NSAIDs.
Selective serotonin reuptake inhibitors (SSRIs)
Since SSRIs impair platelet activation and increase the risk of bleeding, concomitant use of SSRIs with clopidogrel should be done with caution.
Since clopidogrel is metabolized to its active metabolite in part by the CYP2C19 isoenzyme, the use of drugs that inhibit the activity of this enzyme may lead to a decrease in the level of the active metabolite. The clinical significance of this interaction is unknown. As a precaution, it is recommended to avoid the simultaneous use of clopidogrel with drugs that inhibit the CYP2C19 isoenzyme (omeprazole esomeprazole fluvoxamine fluoxetine moclobemide voriconazole fluconazole ticlopidine ciprofloxacin cimetidine carbamazepine oxcarbazepine chloramphenicol). If there is a need to prescribe proton pump inhibitors simultaneously with clopidogrel, drugs with the least inhibition of the CYP2C19 isoenzyme (for example, pantoprazole or lansoprazole) should be used.
There is no evidence that other drugs that reduce gastric acidity such as H2-blockers (except for cimetidine, which is an inhibitor of the CYP2C19 isoenzyme) or antacids affect the antiplatelet properties of clopidogrel.
A number of clinical studies have been conducted with clopidogrel and other concomitantly prescribed drugs to study possible pharmacodynamic and pharmacokinetic interactions which showed that:
no clinically significant pharmacodynamic interaction is observed when clopidogrel is used together with atenolol and/or nifedipine;
simultaneous use of phenobarbital cimetidine and estrogens does not have a significant effect on the pharmacodynamics of clopidogrel;
antacids do not reduce the absorption of clopidogrel;
Phenytoin and tolbutamide can be safely used concomitantly with clopidogrel. It is unlikely that clopidogrel can affect the metabolism of other drugs such as phenytoin and tolbutamide and NSAIDs that are metabolized by the CYP2C19 isoenzyme of the cytochrome P450 group.
Apart from the information presented above, no other drug interaction studies have been conducted. However, patients participating in clinical studies with clopidogrel were simultaneously treated with a number of other drugs including diuretics, beta-blockers, ACE inhibitors, calcium channel blockers, lipid-lowering agents (including insulin), coronary vasodilators, antiepileptic drugs and glycoprotein IIb/IIIa receptor blockers, without evidence of clinically significant adverse interactions.
Overdose
Overdose
Symptoms: an overdose of clopidogrel can lead to prolongation of bleeding time and subsequent hemorrhagic complications in the form of bleeding.
Treatment: If bleeding is detected, appropriate treatment should be given.
An antidote for clopidogrel has not been established.
If rapid correction of prolonged bleeding time is necessary, platelet transfusion is recommended.
Storage conditions
Storage conditions
In a place protected from light at a temperature not exceeding 25 ° C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Do not use after expiration date.
Manufacturer
Manufacturer
Biocom JSC, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | In the dark place at a temperature not exceeding 25 °С. Store out of the reach of children. |
| Manufacturer | Biocom AO, Russia |
| Medication form | pills |
| Brand | Biocom AO |
Other forms…
Related products
Buy Clopidogrel, 75 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.