No products in the cart.
Cereton, 400 mg capsules 14 pcs
€16.59 €13.83
Description
Pharmgroup:
Notropic.
Pharm Action:
Cereton is a nootropic. A central cholinostimulant that contains 40.5% metabolically protected choline. Metabolically protected promotes the release of choline in the brain.
Provides synthesis of acetylcholine and phosphatidylcholine in neuronal membranes, improves blood flow and enhances metabolic processes in the CNS, activates the reticular formation. It increases linear velocity of blood flow on the side of traumatic brain injury, promotes normalization of spatiotemporal characteristics of spontaneous bioelectrical activity of the brain, regress of focal neurological symptoms and restoration of consciousness; it has positive influence on cognitive and behavioral reactions of patients with cerebral vascular diseases (discirculatory encephalopathy and residual effects of cerebral circulatory disorders).
It has preventive and corrective effect on pathogenetic factors of involutional psychoorganic syndrome, changes the phospholipid composition of neuronal membranes and reduces cholinergic activity.
Stimulates dose-dependent release of acetylcholine under physiological conditions; participating in the synthesis of phosphatidylcholine (membrane phospholipid), improves synaptic transmission, neuronal membrane plasticity, receptor function.
It has no effect on the reproductive cycle and has no teratogenic, mutagenic action.
Pharmacokinetics:
When parenteral administration (10 mg/kg) Cereton® predominantly accumulates in the brain, lungs and liver. Absorption is 88%, easily penetrates through the blood-brain barrier (when administered orally, the concentration in the brain is 45% of that in plasma).
The lungs excrete 85% of the drug as carbon dioxide, the remaining amount (15%) is excreted by the kidneys and through the intestine.
Indications
Indications
Use in adults
• Cerebral circulation disorders of ischemic type (acute and recovery periods) and hemorrhagic type (recovery period).
• Psychoorganic syndrome against the background of involutional and degenerative processes of the brain.
• Consequences of cerebrovascular insufficiency or primary and secondary cognitive impairment in the elderly, characterized by memory impairment, confusion, disorientation, decreased motivation and initiative, decreased concentration.
• Behavioral and affective disorders in old age; emotional lability, increased irritability, decreased interest; senile pseudomelancholy.
• Multi-infarct dementia.
Use in children
• Cognitive impairment of mild to moderate severity caused by traumatic brain injury and/or hemorrhagic stroke (including the recovery period and long-term consequences of the above conditions) in children 11 years of age and older (for this dosage form).
Pharmacological effect
Pharmacological effect
CodeATX: M07AX02 Pharmacological properties
Pharmacodynamics
Cereton® is a choline alfoscerate, which is a centrally acting cholinomimetic with a predominant effect on the central nervous system. The drug contains 40.5% choline, released from a compound in the brain; choline is involved in the biosynthesis of acetylcholine (one of the main mediators of nervous excitation). Alphoscerate is biotransformed to glycerophosphate, which is a precursor to phospholipids. Acetylcholine has a positive effect on the transmission of nerve impulses, and glycerophosphate is involved in the synthesis of phosphatidylcholine (membrane phospholipid), resulting in improved membrane elasticity and receptor function.
Pharmacokinetics
Absorption when taken orally – 88%; easily penetrates the blood-brain barrier, accumulates mainly in the brain (concentration in the brain reaches 45% of the level in the blood), lungs and liver; 85% is excreted by the lungs in the form of carbon dioxide, the rest (15%) is excreted by the kidneys and through the intestines.
Does not affect the reproductive cycle, does not have teratogenic or mutagenic effects.
Special instructions
Special instructions
Impact on the ability to drive vehicles and machinery
During the treatment period, care must be taken when driving vehicles and when engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Conditions of release Dispensed by prescription.
Active ingredient
Active ingredient
Choline alphoscerate
Composition
Composition
Composition per capsule
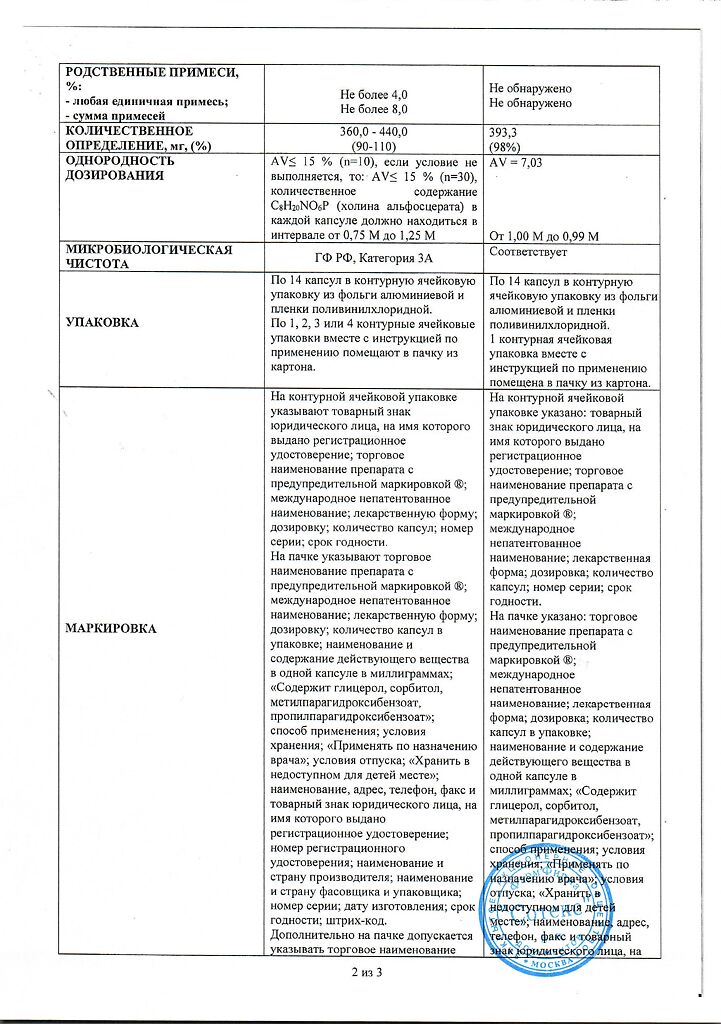
Active ingredient: choline alfoscerate (in terms of 100% substance) – 400 mg; excipients: glycerol, purified water;
soft gelatin capsule: gelatin, sorbitol, glycerol, methyl parahydroxybenzoate, titanium dioxide (E 171), yellow iron oxide dye (E 172), propyl parahydroxybenzoate, purified water.
Pregnancy
Pregnancy
The use of Cereton during pregnancy and breastfeeding is contraindicated.
During treatment with Cereton, you should stop breastfeeding.
Contraindications
Contraindications
• Hypersensitivity to the active substance and/or any excipient of the drug.
• Pregnancy.
• Breastfeeding period.
• Children under 11 years of age (for this dosage form) when used as indicated: mild to moderate cognitive impairment caused by traumatic brain injury or hemorrhagic stroke.
• Children under 18 years of age when used for other indications (due to the lack of data on effectiveness and safety).
Side Effects
Side Effects
From the gastrointestinal tract: nausea (which is mainly a consequence of secondary dopaminergic activation), abdominal pain.
From the nervous system: short-term confusion (in this case it is necessary to reduce the dose of the drug).
The drug is well tolerated even with long-term use.
Overdose
Overdose
Nausea may occur. Treatment: symptomatic therapy.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Artlife LLC, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | PharmFirm Sotex, Russia |
| Medication form | capsules |
| Brand | PharmFirm Sotex |
Other forms…
Related products
Buy Cereton, 400 mg capsules 14 pcs with delivery to USA, UK, Europe and over 120 other countries.