Subtotal: €63.56
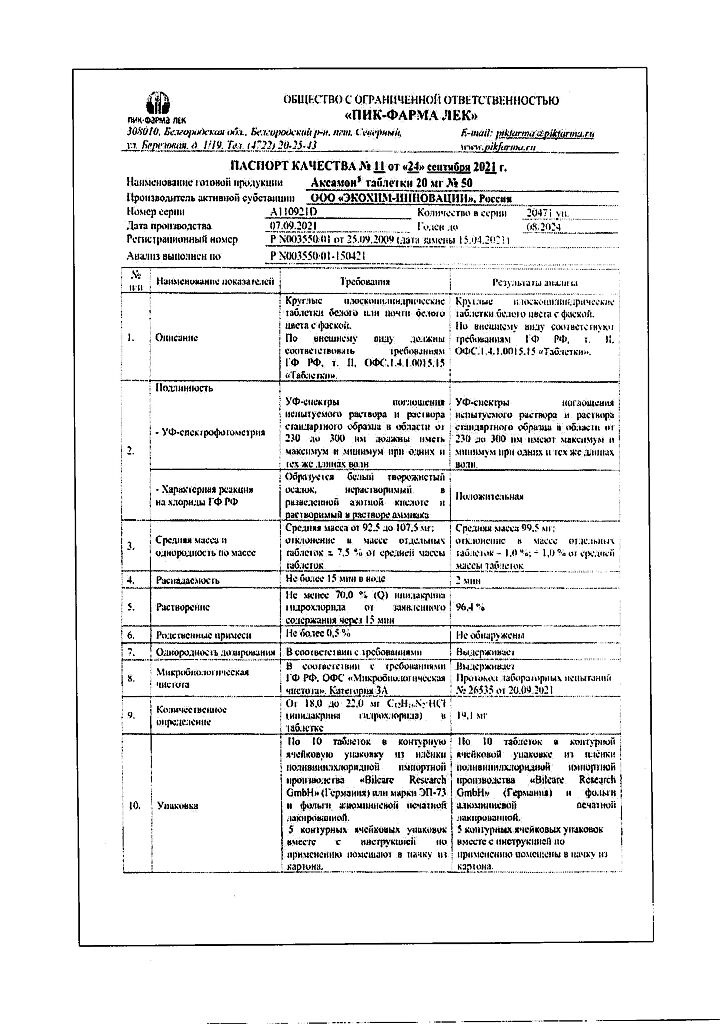
Axamon, tablets 20 mg 50 pcs
€33.82 €31.29
Pharmacodynamics.
A reversible cholinesterase inhibitor. Due to blockade of potassium channels of cell membranes and inhibition of cholinesterase activity it stimulates neuromuscular transmission and conduction of impulses in the nervous system.
It has M- and H-cholinomimetic action. It increases the effect of acetylcholine, serotonin, histamine and oxytocin on smooth muscles which results in increased smooth muscle contractility of organs including GI and bronchi, decreases heart rate, increases salivary glands secretion, myometrium contractile activity and skeletal muscle tone.
It has a stimulating effect on the CNS in combination with some manifestations of sedation; it helps to improve learning and memory.
Pharmacokinetics.
It is rapidly absorbed from the gastrointestinal tract. The maximum plasma concentration is reached after one hour.
40-55% of the active substance is bound to plasma proteins.
The elimination half-life is about 40 minutes. It is metabolized in the liver.
Extraction is through the kidneys (mainly by tubular secretion and only 1/3 of the drug is excreted by glomerular filtration) and also extrarenal (through the gastrointestinal tract).
Indications
Diseases of the peripheral nervous system (neuritis, polyneuritis and polyneuropathy, polyradiculopathy, myasthenia gravis and myasthenic syndrome of various etiologies);
The recovery period of organic lesions of the central nervous system, accompanied by motor disorders, including bulbar paralysis and paresis;
Complex therapy of demyelinating diseases;
Senile dementia, Alzheimer’s disease, encephalopathy of various origins.
Pharmacological effect
Pharmacodynamics.
Reversible cholinesterase inhibitor. Due to the blockade of potassium channels in cell membranes and inhibition of cholinesterase activity, it stimulates neuromuscular transmission and conduction of impulses in the nervous system.
It has M- and N-cholinomimetic effects. Enhances the effect of acetylcholine, serotonin, histamine and oxytocin on smooth muscles, resulting in increased contractility of smooth muscle organs, incl. gastrointestinal tract and bronchi, reduces heart rate, increases the secretion of the salivary glands, the contractile activity of the myometrium, and the tone of skeletal muscles.
Has a stimulating effect on the central nervous system in combination with individual manifestations of sedation; helps improve learning and memory.
Pharmacokinetics.
Rapidly absorbed from the gastrointestinal tract. The maximum concentration in blood plasma is reached after one hour.
40-55% of the active substance binds to blood plasma proteins.
The half-life is approximately 40 minutes. Metabolized in the liver.
Excretion is carried out through the kidneys (mainly by tubular secretion and only 1/3 of the drug is excreted by glomerular filtration), as well as extrarenally (through the gastrointestinal tract).
Special instructions
During treatment, you should refrain from driving a car, other vehicles and machinery, as well as from engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Ipidacrine
Composition
1 tablet contains:
active substance:
ipidacrine hydrochloride monohydrate (Axamon) 20 mg (in terms of ipidacrine hydrochloride);
excipients:
ludipress (lactose monohydrate 93%, povidone 3.5%, crospovidone 3.5%) 65 mg,
sodium carboxymethyl starch 14 mg,
calcium stearate 1 mg.
Pregnancy
The use of the drug increases the tone of the uterus and can lead to premature birth, therefore ipidacrine is contraindicated during pregnancy.
There are no data on the use of the drug during breastfeeding.
Contraindications
Hypersensitivity to any of the components of the drug, epilepsy, extrapyramidal disorders with hyperkinesis, angina pectoris, severe bradycardia, bronchial asthma, mechanical obstruction of the intestine or urinary tract, vestibular disorders, peptic ulcer of the stomach or duodenum in the acute stage, galactose intolerance, lactase deficiency or glucose-galactose malabsorption, pregnancy and lactation period, children under 18 years of age.
With caution in case of: gastric and duodenal ulcers in remission, thyrotoxicosis, intracardiac conduction disorders; history of obstructive respiratory diseases or acute respiratory diseases.
Side Effects
Caused by stimulation of m-cholinergic receptors: salivation, increased sweating, nausea, diarrhea, jaundice, bradycardia, epigastric pain, increased secretion of bronchial secretions, bronchospasm, convulsions, increased uterine tone.
Salivation and bradycardia can be reduced
m-anticholinergics: atropine, trihexyphenidyl (cyclodol), metocinium iodide (metacin), etc.
Less commonly, after using higher doses, dizziness, ataxia, headache, vomiting, general weakness, drowsiness, and skin reactions (itching, rash) were observed. In these cases, reduce the dose or interrupt the drug for a short time (for 1-2 days).
Interaction
Axamon® enhances the sedative effect in combination with drugs that depress the central nervous system.
The action and side effects are enhanced when used together with other cholinesterase inhibitors and m-cholinergic mimetic drugs.
In patients with myasthenia gravis, the risk of developing a cholinergic crisis increases if Axamon is used simultaneously with other cholinergic drugs.
The risk of developing bradycardia increases if beta-blockers were used before starting treatment with Axamon®.
Reduces the inhibitory effect on neuromuscular transmission and the conduction of excitation along peripheral nerves by local anesthetics, aminoglycosides, and potassium chloride.
Axamon® can be used in combination with nootropic drugs.
Alcohol increases the side effects of the drug.
Overdose
Symptoms: decreased appetite, bronchospasm, lacrimation, increased sweating, constriction of the pupils, nystagmus, increased peristalsis of the gastrointestinal tract, spontaneous bowel movements and urination, vomiting, jaundice, bradycardia, intracardiac conduction disorders, arrhythmias, decreased blood pressure, restlessness, anxiety, agitation, feelings of fear, ataxia, convulsions, coma, speech disorders, drowsiness and general weakness.
Treatment: use of m-anticholinergic drugs (atropine, cyclodol, metacin, etc.), symptomatic therapy.
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
3 years
Manufacturer
Pik-Pharma, Russia
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Pik-Pharma, Russia |
| Medication form | pills |
| Brand | Pik-Pharma |
Other forms…
Related products
Buy Axamon, tablets 20 mg 50 pcs with delivery to USA, UK, Europe and over 120 other countries.