Subtotal: €4.01
Aqualor Baby, 150 ml
€12.45 €10.38
SARS, Sinusitis, Runny nose (rhinitis), Allergic rhinitis, Angina, Respiratory infections, Laryngitis, Pharyngitis, Adenoiditis, Influenza, Stomatitis, Haymoritis – Prevention and comprehensive treatment of acute and chronic inflammatory diseases of the nasal cavity, paranasal sinuses and nasopharynx (infectious, allergic, atrophic):
– acute and chronic rhinitis (runny nose)
– acute and chronic sinusitis (including sinusitis).Acute and chronic rhinitis (runny nose)
– Allergic and vasomotor rhinitis
– Subatrophic rhinitis (dry mucous membranes)
– Prevention and complex treatment of respiratory infections (acute respiratory infections, etc.).
– Daily hygiene of the nasal cavity and nasopharynx
– State after surgical interventions in the nasal cavity and paranasal sinuses
Indications
Area of application: otorhinolaryngology. The product is intended for the prevention and complex treatment of ENT diseases in children from birth and adults through irrigation and rinsing of the nasal cavity.
The product is used for:
• prevention and complex treatment of acute and chronic inflammatory diseases of the nasal cavity, paranasal sinuses and nasopharynx (infectious, allergic, atrophic):
acute and chronic rhinitis (runny nose);
acute and chronic sinusitis (including sinusitis);
acute and chronic adenoiditis;
allergic and vasomotor rhinitis;
subatrophic rhinitis (dry mucous membrane);
• prevention and complex treatment of respiratory infections (flu, ARVI, etc.);
• daily hygiene of the nasal cavity and nasopharynx;
• prevention and complex treatment of conditions after surgical interventions in the nasal cavity and paranasal sinuses;
The product is intended from birth.
Special instructions
Inspect the product and packaging before use. Do not use the product if the integrity of the packaging, the medical device itself, or the nozzle bridge is damaged. When you press the nozzle for the first time, the integrity of the jumper is broken (this is indicated by a click).
To ensure ideal product quality and functioning of the dosing system, do not disassemble the medical device (for example, remove the anatomical attachment from the balloon).
Active components
Natural sterile sea water isotonic
Composition
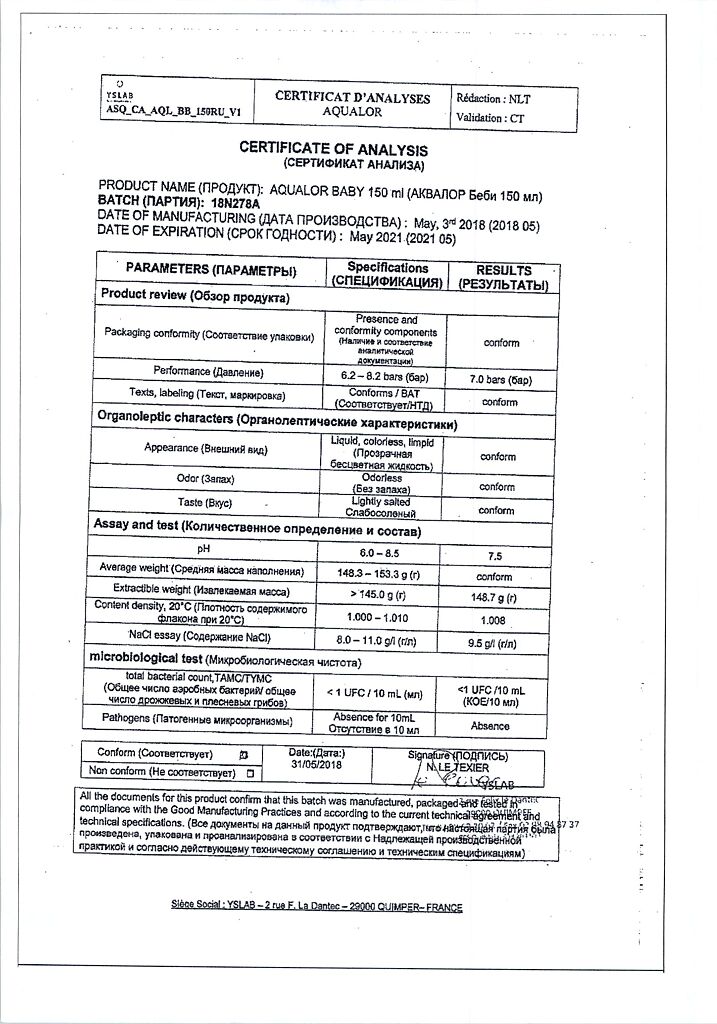
Isotonic seawater solution (NaCl concentration 8 – 11 g/l),
containing natural sea water, purified water.
Sea water is collected from ecologically clean areas off the coast of Brittany (France) and Lyseshil (Sweden). Sea water contains ions of sodium, potassium, zinc, selenium, magnesium, calcium, iodine, chlorine, bromine.
pH 6.0 – 8.5
Does not contain preservatives.
Pregnancy
Pregnancy and breastfeeding are not contraindications.
Contraindications
Individual intolerance to the components of a medical device.
Side Effects
Not identified.
Specifications
Means for irrigation and rinsing of the nasal cavity for children and adults “Aqualor® baby” 150 ml according to TU 32.50.50-009-00480081-2017 (hereinafter referred to as the means) is an aerosol in an aluminum pressure cylinder with a polyethylene anatomical nozzle for the nose, creating a “soft shower” spray, with a restrictive ring and with polypropylene cap. A four-layer bag with contents is placed inside the cylinder, equipped with a special one-way valve, which maintains the sterility of the contents throughout the entire period of use by preventing reverse flow of the contents and preventing ambient air from entering the bag. The nozzle has a jumper to control pressing on the aerosol valve. Not a medicine.
Transparent, colorless, odorless liquid
The spray pattern is “soft shower”.
The nozzle is equipped with a restrictive ring that prevents deep penetration and traumatization of the child’s nasal mucosa.
Sterile. Maintains sterility throughout the entire period of use.
Aluminum pressure cylinder equipped with a polyethylene anatomical nozzle for the nose and a polypropylene cap in a cardboard box with instructions for use of the medical device
Prescribing
The product is intended to remove mucus, reduce secretions, soften and remove crusts, and moisturize the nasal mucosa. It has a beneficial effect on the mucous membrane of the nasal cavity and nasopharynx, helps reduce nasal congestion and restore nasal breathing. The salts contained in sea water help thin the mucus and normalize its production in the goblet cells of the mucous membrane. Calcium and magnesium ions contained in salt significantly improve the functioning of ciliated epithelial cells of the nasal mucosa, normalize the rheological properties of mucus, and increase resistance to the introduction of viruses and bacteria. Iodine and sodium chloride have antiseptic properties. Zinc and selenium ions create conditions for the activation of local immunity of the nasal mucosa and paranasal sinuses.
After rinsing the nasal cavity with the product, the therapeutic effectiveness of drugs applied to the mucous membrane of the nasal cavity increases, the duration of respiratory diseases is reduced, and the risk of infection spreading to the paranasal sinuses and ear cavity is reduced. Helps remove any pathogens (viral, bacterial, fungal) from the mucous membrane of the nasal cavity and nasopharynx without disturbing the biocenosis of the mucous membrane.
The product is intended for individual use at home and in medical and treatment-and-prophylactic medical institutions.
Storage conditions
Store and use at temperatures from + 5 °C to + 25 °C. Keep out of the reach of children. Keep away from heat.
The product is transported by any type of land and air transport in accordance with the cargo transportation rules in force for this type of transport at temperatures from + 5 ° C to + 25 ° C.
The product belongs to class A of the classification of medical waste in accordance with the Sanitary and Epidemiological Rules and Standards SanPiN 2.1.3684-21. Accounting and control of the movement of class A waste is carried out in accordance with the requirements of the legislation of the Russian Federation. Waste collection is provided by the distributor (contracted service organizations or contracted service technicians).
Shelf life
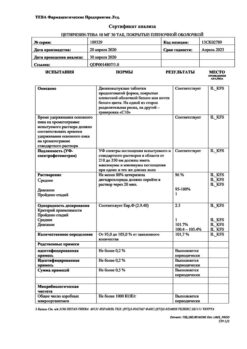
Shelf life – 3 years. The expiration date is indicated on the bottom of the cylinder.
Manufacturer
Nizhpharm JSC, Russia
| Shelf life | Shelf life – 3 years. Use until the date indicated on the package. |
|---|---|
| Conditions of storage | Store in dry place protected from direct sunlight at the temperature from 5 °С to 25 °С. Keep out of reach of children. Nasal irrigation and cleansing agent for children and adults "Aqualor baby mini", "Aqualor baby", "Aqualor soft mini", "Aqualor norm mini", "Aqualor norm" is transported by all means of transport in covered vehicles in compliance with transport regulations in force for this type of transport at temperature from 1 °С to 40 °С. Nasal irrigation and cleansing agent for children and adults "Aqualor baby mini", "Aqualor baby", "Aqualor soft mini", "Aqualor norm mini", "Aqualor norm" – is classified as A-class medical waste according to Sanitary and epidemiological rules and regulations SanPiN 2.1.7.2790-10. Accounting and control of the movement of class A wastes is carried out in accordance with the requirements of the legislation of the Russian Federation. Collection of waste is provided by the distributor (contractual service organizations or contractual service technicians). |
| Manufacturer | IS LAB, France |
| Medication form | nasal spray |
| Brand | IS LAB |
Other forms…
Related products
Buy Aqualor Baby, 150 ml with delivery to USA, UK, Europe and over 120 other countries.