No products in the cart.
Andipal, tablets 10 pcs
€5.37 €3.60
Description
Pharmacotherapeutic group: analgesic (analgesic non-narcotic spasmolytic barbiturate).
ATC code: M02BB52.
Pharmacological properties
Combination drug. The combination of the drug components leads to mutual enhancement of their pharmacological action. Metamizole sodium is a pyrazolone derivative with analgesic and antipyretic effect. Bendazole (dibazole) is a vasodilator; it has a vasodilator effect, stimulates spinal cord function.
It has a direct antispasmodic effect on smooth muscles of blood vessels and internal organs. Facilitates synaptic transmission in the spinal cord. Papaverine hydrochloride is an antispasmodic agent, has a hypotensive effect, reduces tone and relaxes smooth muscles of internal organs and blood vessels. Phenobarbital in small doses has a sedative effect and enhances the effect of other components.
Indications
Indications
Pain syndrome (mild or moderate) with spasms of smooth muscles of internal organs:
renal colic, biliary colic, intestinal colic;
biliary dyskinesia, postcholecystectomy syndrome;
spasm of the ureters and bladder;
algodismenorrhea.
Spasm of cerebral vessels, migraine.
As an auxiliary drug: pain syndrome after surgery and diagnostic procedures.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: analgesic (analgesic non-narcotic drug antispasmodic barbiturate).
ATX code: M02BB52.
Pharmacological properties
Combined drug. The combination of the components of the drug leads to a mutual enhancement of their pharmacological action. Metamizole sodium is a pyrazolone derivative that has an analgesic and antipyretic effect. Bendazole (dibazole) is a vasodilator; has a vasodilating effect, stimulates the function of the spinal cord.
It has a direct antispasmodic effect on the smooth muscles of blood vessels and internal organs. Facilitates synaptic transmission in the spinal cord. Papaverine hydrochloride is an antispasmodic, has a hypotensive effect, reduces tone and relaxes the smooth muscles of internal organs and blood vessels. Phenobarbital, in small doses, has a sedative effect and enhances the effect of other components.
Special instructions
Special instructions
With long-term (more than 7 days) use, monitoring of the peripheral blood picture and the functional state of the liver is necessary.
If there is no effect within 3 days, you should stop taking the drug and consult a doctor.
During treatment, you should avoid engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Children and adolescents under 18 years of age should use the drug only as prescribed by a doctor.
Active ingredient
Active ingredient
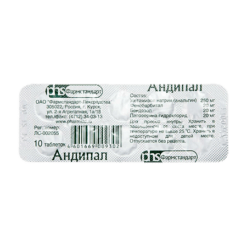
Bendazole, Metamizole sodium, Papaverine, Phenobarbital
Composition
Composition
For one tablet:
Active substances:
metamizole sodium (analgin) – 250 mg,
phenobarbital – 20 mg,
bendazole (dibazole) – 20 mg,
papaverine hydrochloride – 20 mg.
Excipients:
potato starch – 46 mg,
talc – 7 mg,
stearic acid – 3 mg,
calcium stearate – 4 mg.
Pregnancy
Pregnancy
The drug is contraindicated during pregnancy and breastfeeding.
Pregnancy
Data on the use of the combination drug Andipal during pregnancy are limited. Metamizole sodium, which is part of the drug, penetrates the placenta. According to the results of preclinical studies, the teratogenic effect of metamizole sodium was not detected. Despite the fact that metamizole sodium weakly inhibits the synthesis of prostaglandins, premature (intrauterine) closure of the ductus arteriosus as well as perinatal complications caused by impaired platelet aggregation in the mother or newborn cannot be ruled out.
Breast-feeding
Metamizole sodium metabolites pass into breast milk; therefore, when using the drug Andipal, as well as within 48 hours after taking the last dose, you must stop breastfeeding.
Contraindications
Contraindications
Hypersensitivity (including to pyrazolone derivatives);
inhibition of bone marrow hematopoiesis;
severe liver and/or kidney failure;
deficiency of glucose-6-phosphate dehydrogenase;
children under 8 years of age, pregnancy, lactation, tachyarrhythmias, severe angina, collapse, decompensated chronic heart failure;
angle-closure glaucoma;
prostatic hyperplasia;
intestinal obstruction, megacolon.
Side Effects
Side Effects
Allergic reactions.
From the central nervous system: drowsiness, decreased speed of psychomotor reactions.
From the gastrointestinal tract: nausea, constipation.
From the cardiovascular system: arterial hypotension.
With long-term use: leukopenia, agranulocytosis, impaired liver and kidney function.
Interaction
Interaction
Combination with nitrates (nitroglycerin, nitrosorbide, sustak, etc.), calcium channel blockers (nifedipine, Corinfar, etc.), beta-blockers (anaprilin, metoprolol, talinolol, etc.), ganglion blockers (pentamine, etc.), diuretics (furosemide, hypothiazide, etc.), myotropic antispasmodics (dipyridamole, aminophylline, etc.) enhances the hypotensive effect of these drugs.
Concomitant use with other non-narcotic analgesics can lead to mutual enhancement of toxic effects. Combined use with adsorbents, astringents and enveloping agents reduces the absorption of the drug in the gastrointestinal tract.
Overdose
Overdose
Andipal
Symptoms of overdose: an overdose of the drug is due to the properties of its constituent components. In case of an overdose, severe drowsiness, dizziness, and a collapsible state occur.
Treatment: first aid – gastric lavage, intake of activated carbon. Symptomatic therapy aimed at maintaining vital functions. Treatment of intoxication as well as prevention of serious complications require intensive medical supervision and treatment.
Metamizole sodium
Symptoms of overdose: acute overdose is manifested by nausea, vomiting, abdominal pain, impaired renal function/acute renal failure (for example, as a manifestation of interstitial nephritis) and rarely by symptoms of the central nervous system (coma, convulsions) and a decrease in blood pressure leading to tachycardia and shock. In high overdoses, excretion of rubazonic acid may turn the urine red.
Treatment: no specific antidote is known. In case of a recent overdose, in order to limit the entry of the drug into the body, primary detoxification (for example, gastric lavage) or sorption therapy (for example, activated carbon) is performed. The main metabolite (4N-methylaminoantipyrine) is removed by hemodialysis, hemofiltration, hemoperfusion and plasmafiltration. Treatment of overdose, as well as prevention of serious complications, may require general and special intensive medical supervision and treatment.
Phenobarbital
Symptoms of overdose: nystagmus ataxia headache lethargy slurred speech severe weakness decreased or loss of reflexes agitation increased or decreased body temperature respiratory depression shortness of breath decreased blood pressure constriction of the pupils (alternating with paralytic dilation) oliguria tachy- or bradycardia cyanosis confusion cessation of electrical activity of the brain pulmonary edema coma later – pneumonia cardiac arrhythmias insufficiency; when taking 2-10 g – death; in case of chronic toxicity – irritability, weakened ability to critically assess sleep disturbances, confusion.
Treatment: there is no specific antidote. Gastric lavage, intake of activated charcoal, detoxification therapy, symptomatic treatment, maintenance of vital body functions.
Bendazole
Symptoms of overdose: There are no data on cases of overdose. The most likely adverse event would be a marked decrease in blood pressure.
Treatment: if there is a pronounced decrease in blood pressure, place the patient in a “lying” position with raised lower limbs and carry out symptomatic therapy.
Papaverine hydrochloride
Symptoms of overdose: diplopia (double vision), weakness, decreased blood pressure.
Treatment: symptomatic (maintaining blood pressure).
Storage conditions
Storage conditions
In a dry place, protected from light and out of reach of children.
Shelf life
Shelf life
2 years 6 months.
The drug should not be used after the expiration date.
Manufacturer
Manufacturer
Update of PFC JSC, Russia
Additional information
| Shelf life | 2 years 6 months. The drug must not be used after the expiration date. |
|---|---|
| Conditions of storage | In a dry place, protected from light and out of the reach of children. |
| Manufacturer | Update PFC AO, Russia |
| Medication form | pills |
| Brand | Update PFC AO |
Other forms…
Related products
Buy Andipal, tablets 10 pcs with delivery to USA, UK, Europe and over 120 other countries.