No products in the cart.
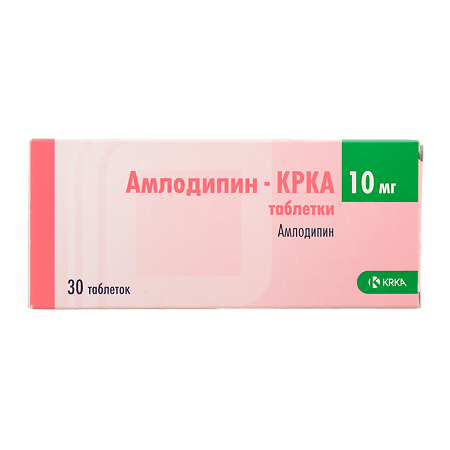
Amlodipine-CRCA, tablets 10 mg 30 pcs
€5.90 €4.92
Description
Stenocardia, Edema, Heart pain, Blood pressure, Hypertension (high blood pressure)
- Arterial hypertension (both in monotherapy and in combination with other hypotensive agents).
- Stable angina pectoris and vasospastic angina pectoris (Prinzmetal angina) (both in monotherapy and in combination with other antianginal agents).
Active ingredient
Active ingredient
Composition
Composition
1 tablet 5 mg/10 mg contains:
Active substance:
Amlodipine maleate 6.42 mg/12.84 mg, equivalent to amlodipine 5.00 mg/10.00 mg
Auxiliary substances:
Microcrystalline cellulose, pregelatinized starch, sodium carboxymethyl starch, colloidal silicon dioxide, magnesium stearate
.
How to take, the dosage
How to take, the dosage
Orally, once daily with the required amount of water (100 ml).
Arterial hypertension, stable angina pectoris and vasospastic angina pectoris
Arterial hypertension, stable angina pectoris and vasospastic angina pectoris: justify;”>In arterial hypertension and angina pectoris, the usual starting dose is 5 mg per day. Depending on the therapeutic response, the dose may be increased to a maximum daily dose of 10 mg. Usually, it is recommended to increase the dose of amlodipine not earlier than after 7-14 days of treatment. However, if necessary, faster increase of amlodipine dose is possible with regular patient monitoring.
In patients with low body weight, short stature, or moderate hepatic impairment, the recommended starting dose of amlodipine is 2.5 mg daily.
Simultaneous use with other hypotensive and antianginal drugs
No dose adjustment is required for amlodipine when used concomitantly with thiazide diuretics, beta-adrenoblockers and ACE inhibitors.
When amlodipine is indicated as adjunctive therapy in patients receiving other hypotensive agents, the recommended starting dose is 2.5 mg daily.
Special patient groups
Elderly patients
It is recommended to use the drug in usual doses, no change in amlodipine dose is required.
Patients with impaired liver function
Although the T1/2 of amlodipine, like all BMCCs, is increased in patients with mild hepatic dysfunction, no dose adjustment is usually required. In patients with moderate hepatic impairment the recommended starting dose of amlodipine is 2.5 mg daily.
Patients with impaired renal function
The use of amlodipine in usual doses is recommended.
Interaction
Interaction
Amlodipine can be safely used for therapy of arterial hypertension together with thiazide diuretics, alpha-adrenoblockers, beta-adrenoblockers or ACE inhibitors. In patients with stable angina pectoris amlodipine can be combined with other antianginal agents such as sustained- and short-acting nitrates and beta-adrenoblockers.
Amlodipine has not been found to interact clinically with non-steroidal anti-inflammatory drugs (NSAIDs) including indomethacin unlike other DMARDs.
The antianginal and antihypertensive effects of PBMCs may be enhanced when used concomitantly with thiazide and loop diuretics, ACE inhibitors, beta-adrenoblockers and nitrates as well as their antihypertensive effects when used with alpha 1-adrenoblockers and neuroleptics.
There have been cases of ventricular fibrillation with collapse and death in laboratory animals with verapamil and intravenous dantrolene accompanied by hyperkalemia. Due to the risk of hyperkalemia concomitant use of PBMCs including amlodipine and dantrolene should be avoided in patients with malignant hyperthermia as well as during treatment of malignant hyperthermia.
Although negative inotropic effects have not generally been observed in studies of amlodipine, however, some BMCCs may increase the severity of the negative inotropic effects of antiarrhythmic agents that cause QT interval prolongation (eg, amiodarone and quinidine).
Amlodipine may also be safely used concomitantly with oral antibiotics and hypoglycemic agents.
A single use of 100 mg sildenafil in patients with essential hypertension does not influence pharmacokinetic parameters of amlodipine.
Repeated use of amlodipine in dose of 10 mg and atorvastatin in dose of 80 mg is not accompanied by significant changes of pharmacokinetic parameters of atorvastatin.
Simvastatin: Simultaneous repeated use of amlodipine in dose of 10 mg and simvastatin in dose of 80 mg leads to increase in simvastatin exposure by 77 %. In such cases the simvastatin dose should be limited to 20 mg.
Ethanol (beverages containing alcohol): Amlodipine at a single and repeated use of 10 mg dose does not affect the pharmacokinetics of ethanol.
Antiviral agents (ritonavir):increase plasma concentrations of PBMCs, including amlodipine.
Neuroleptics and isoflurane:increase the antihypertensive effect of dihydropyridine derivatives.
Calcium preparations:can decrease the effect of BMCCs.
Simultaneous use of PBMCs with lithium preparations (no data for amlodipine) may increase their neurotoxicity (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus).
Studies on concomitant use of amlodipine and cyclosporine in healthy volunteers and all patient groups, except for patients after kidney transplantation, have not been conducted. Various studies on the interaction of amlodipine with cyclosporine in patients after kidney transplantation show that the use of this combination may either lead to no effect, or increase the minimum concentration of cyclosporine to varying degrees up to 40%. These data should be taken into account and cyclosporine concentrations should be monitored in this group of patients when concomitant use of cyclosporine and amlodipine.
It does not affect the serum digoxin concentration and its renal clearance.
It does not significantly affect the effect of warfarin (prothrombin time index).
Cimetidine:does not affect the pharmacokinetics of amlodipine.
In invitro studies, amlodipine does not affect the plasma protein binding of digoxin, phenytoin, warfarin and indomethacin.
Grapefruit juice:Concomitant administration of 240 mg grapefruit juice and 10 mg amlodipine orally is not accompanied by significant changes in amlodipine pharmacokinetics. However, it is not recommended to use grapefruit juice and amlodipine concomitantly because amlodipine bioavailability may be increased with genetic polymorphism of CYP3A4 isoenzyme and thus the antihypertensive effect may be enhanced.
Aluminum- or magnesium-containing antacids:their single administration has no significant effect on amlodipine pharmacokinetics.
Inhibitors of isoenzyme CYP3A4: concomitant use of diltiazem at a dose of 180 mg and amlodipine at a dose of 5 mg in patients 69 to 87 years of age with arterial hypertension showed a 57% increase in systemic exposure to amlodipine. Concomitant use of amlodipine and erythromycin in healthy volunteers (18 to 43 years) does not result in significant changes in amlodipine exposure (increase in area under the curve “concentration-time” (AUC) by 22%). Although the clinical significance of these effects is not entirely clear, they may be more pronounced in elderly patients.
Powerful inhibitors of CYP3A4 isoenzyme (e.g., ketoconazole, itraconazole) may increase the plasma concentration of amlodipine to a greater extent than diltiazem. Caution should be exercised when using amlodipine and CYP3A4 isoenzyme inhibitors.
Clarithromycin:patients taking clarithromycin (CYP3A4 inhibitor) and amlodipine concomitantly have an increased risk of BP decrease. Patients taking this combination are recommended to be under close medical supervision.
Inductors of isoenzyme CYP3A4: concomitant use of CYP3A4 isoenzyme inducers may alter the plasma concentration of amlodipine. Therefore it is necessary to monitor BP and adjust the dose of drugs taken both during and after their concomitant use (including rifampicin, St. John’s Wort preparations).
Tacrolimus:concomitant use with amlodipine carries the risk of increased plasma concentrations of tacrolimus. To avoid tacrolimus toxicity when used concomitantly with amlodipine, tacrolimus plasma concentrations in patients should be monitored and the tacrolimus dose should be adjusted if necessary.
Inhibitors of the mechanistic target for rapamycin in mammals (mTOR): MTOR inhibitors, such as sirolimus, temsirolimus, and everolimus, are substrates of the CYP3A isoenzyme. Amlodipine is a weak inhibitor of CYP3A isoenzyme. When used concomitantly with mTOR inhibitors, amlodipine may increase their exposure.
Special Instructions
Special Instructions
Impaired liver function, sinus node weakness syndrome (marked bradycardia, tachycardia), CHF of non-ischemic etiology of NYHA functional class III-IV, mild to moderate degree of arterial hypotension, aortic stenosis, Mitral stenosis, hypertrophic obstructive cardiomyopathy (HACMI), acute myocardial infarction (and within 1 month after myocardial infarction), use in elderly patients, pregnancy and breast-feeding (see sect. section “Use in pregnancy and breastfeeding”), concomitant use with inhibitors or inducers of CYP3A4 isoenzyme.
It is contraindicated under 18 years of age (effectiveness and safety have not been studied).
Elderly patients
It is recommended to use the drug in usual doses; the amlodipine dose should not be changed.
Patients with impaired liver function
Although the T1/2 of amlodipine, like all BMCCs, is increased in patients with mild hepatic dysfunction, no dose adjustment is usually required. In patients with moderate hepatic impairment the recommended starting dose of amlodipine is 2.5 mg daily.
Patients with impaired renal function
It is recommended to use amlodipine in usual doses.
Cardiovascular disease
The efficacy and safety of amlodipine in hypertensive crisis have not been established.
In acute myocardial infarction amlodipine can be used only after hemodynamic stabilization.
In rare cases, patients with coronary heart disease (especially with severe obstructive coronary artery disease) have seen an increase in frequency, duration and/or severity of angina attacks after initiation of BMCC or after increasing their dosage.
Although in general BMCC should be used with caution in patients with CHF, amlodipine did not increase mortality or the incidence of cardiovascular complications in patients with CHF in short- and long-term clinical trials. Against the background of amlodipine use in patients with CHF (functional class III and IV according to NYHA classification) of non-ischemic genesis the increase of pulmonary edema development rate was observed, despite the absence of signs of cardiac insufficiency progression.
Aortic stenosis, mitral stenosis, GOCMP
As with all drugs with vasodilating effects, amlodipine should be used with caution in patients with aortic stenosis, mitral stenosis, or GOCMP. In patients with left ventricular outflow tract obstruction (e.g. severe aortic stenosis) the drug is contraindicated.
Cancellation syndrome
Although there is no withdrawal syndrome in BMCCs, it is advisable to discontinue amlodipine treatment by gradually reducing the dose of the drug. Amlodipine does not prevent abrupt withdrawal from beta-adrenoblockers.
Peripheral edema
Mild to moderate peripheral edema was the most common adverse event reported with amlodipine in clinical trials. The incidence of peripheral edema increased with increasing dose (with amlodipine 2.5 mg, 5 mg and 10 mg daily, edema occurred in 1.8%, 3% and 10.8% of patients, respectively). Peripheral edema associated with amlodipine use should be carefully differentiated from symptoms of progression of left ventricular heart failure.
Liver function impairment
Controlled studies in patients with liver function impairment have not been performed. In a small number of patients with mild to moderate hepatic impairment, increased T1/2 amlodipine was noted. Patients with hepatic impairment should be under medical supervision if amlodipine should be used. In some cases (e.g., in moderate hepatic insufficiency) a lower initial dose of amlodipine (2.5 mg per day) is recommended.
Elderly patients
In elderly patients, T1/2 may be increased and amlodipine clearance decreased. In clinical trials, the incidence of adverse events in patients aged >65 years was approximately 6% higher than in younger patients. No change of amlodipine doses is required, but more careful monitoring of patients in this category is necessary.
Other
During amlodipine therapy, body weight and dietary salt intake should be controlled.
It is necessary to maintain dental hygiene and see a dentist (to prevent soreness, bleeding, and gum hyperplasia).
There have been no reports of the effect of Amlodipine- KRKA on driving or operating machinery. Nevertheless, drowsiness and dizziness may occur in some patients, mainly at the beginning of treatment. If they occur, the patient should exercise caution when driving a car and operating complex mechanisms.
Synopsis
Synopsis
Circular, biconvex tablets are white or almost white with a bevel and a rib on one side.
Contraindications
Contraindications
Side effects
Side effects
The frequency classification of side effects recommended by the World Health Organization (WHO):
very frequently â¥1/10
often ⥠1/100 to < 1/10
infrequently from ⥠1/1000 to < 1/100
rarely from ⥠1/10000 to < 1/1000
very rarely from < 1/10000
frequency is unknown cannot be estimated from available data.
Blood and lymphatic system disorders:
very rare: thrombocytopenic purpura, leukopenia, thrombocytopenia.
Immune system disorders:
infrequent: urticaria, allergic reactions;
very rare: angioedema.
Disorders of metabolism and nutrition:
Disorders of metabolism and nutrition:
infrequent: increase/decrease in body weight;
very rare: hyperglycemia.
Mental disorders:
infrequent: unusual dreams, anxiety, depression, depersonalization, insomnia, mood changes.
Nervous system disorders:
often: headache, dizziness, increased fatigue, drowsiness;
infrequently: Asthenia, hyperesthesia, paresthesia, peripheral neuropathy, tremor, mood lability, hyperexcitability, dysgeusia (perversion of taste);
very rarely: migraine, apathy, agitation, ataxia, amnesia;
frequency unknown: extrapyramidal disorders.
Visual disorders:
infrequent: diplopia, accommodation disorders, xerophthalmia, conjunctivitis, eye pain, visual disturbances.
Hearing organ and labyrinth disorders:
infrequent: tinnitus, vertigo.
Cardiac disorders:
often: palpitations;
very rarely: cardiac rhythm disturbances (including bradycardia, ventricular tachycardia, and atrial fibrillation), development or worsening of the course of CHF, myocardial infarction, chest pain, pulmonary edema.
vascular disorders:
very often: peripheral edema (ankles and feet);
often: blood “rushes” to the skin of the face;
infrequently: excessive BP decrease, orthostatic hypotension;
very rarely: vasculitis.
Disorders of the respiratory system, thoracic and mediastinal organs:
infrequently: shortness of breath, rhinitis, nasal bleeding;
very rarely: cough.
Gastrointestinal tract disorders:
often: abdominal pain, nausea;
infrequently: vomiting, constipation, diarrhea, flatulence, dyspepsia, anorexia, dry oral mucosa, bloating;
rarely: gum hyperplasia, increased appetite;
very rarely: pancreatitis, gastritis.
Liver and biliary tract disorders:
incidence unknown: hepatitis, jaundice (due to cholestasis), hyperbilirubinemia, increased activity of “hepatic” transaminases in blood plasma.
Skin and subcutaneous tissue disorders:
infrequent: skin itching, skin rash (including erythematous, maculopapular rash), increased sweating;
rarely: dermatitis;
very rare: erythema multiforme, alopecia, xeroderma, discoloration of skin pigmentation.
Muscular and connective tissue disorders:
infrequent: arthralgia, muscle cramps, myalgia, back pain, arthrosis;
rare: myasthenia, muscle weakness.
Kidney and urinary tract disorders:
infrequent: Painful urination, nycturia, rapid urination;
very rarely: dysuria, polyuria.
Gender and mammary gland disorders:
infrequent: gynecomastia, erectile dysfunction, sexual dysfunction (in men and women).
General disorders and disorders at the site of administration:
infrequently: chills, thirst, general malaise, asthenia;
very rarely: syncope, parosmia.
Overdose
Overdose
Symptoms:excessive peripheral vasodilation with strong and possibly prolonged BP decrease, collapse, shock.
treatment:gastric lavage, administration of activated charcoal, maintenance of cardiovascular function, control of heart and lung function parameters, elevated (above head level) position of lower extremities, control of circulating blood volume (CBC) and diuresis. To restore vascular tone – use vasoconstrictors (if there are no contraindications for their use), to eliminate the effects of calcium channel blockade – intravenous injection of calcium gluconate. Hemodialysis is ineffective.
Pregnancy use
Pregnancy use
The safety of Amlodipine – KRKA administration during pregnancy and breastfeeding has not been established, therefore the use during pregnancy and breastfeeding is possible only when the expected benefits to the mother exceed the possible risk to the fetus and newborn.
Experience of use shows that amlodipine is excreted in women’s breast milk. The mean milk/plasma ratio was 0.85 among 31 lactating women with pregnancy-related arterial hypertension receiving amlodipine at an initial dosage of 5 mg daily. The dosage was adjusted as necessary (based on mean daily dose and weight: 6 mg and 98.7 mcg/kg, respectively). The estimated daily dose of amlodipine received by the infant via breast milk is 4.17 mcg/kg.
Reversible biochemical changes in the sperm head were observed in some patients when using calcium channel blockers. Clinical data on the potential effect of amlodipine on fertility are insufficient. In a study in rats, adverse effects on fertility in males have been reported.
Similarities
Similarities
Additional information
| Weight | 0.028 kg |
|---|---|
| Shelf life | 4 years. Do not use the drug after the expiration date. tab-stops: 45.8pt 91.6pt 137.4pt 183.2pt 229.0pt 274.8pt 320.6pt 366.4pt 412.2pt 458.0pt 503.8pt 549.6pt 595.4pt 641.2pt 687.0pt 732.8pt;"> |
| Conditions of storage | Store at 25°C or less, in original container. Store out of reach of children. |
| Manufacturer | KRKA-RUS, Russia |
| Medication form | pills |
| Brand | KRKA-RUS |
Other forms…
Related products
Buy Amlodipine-CRCA, tablets 10 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.