No products in the cart.
Amigrenin, 100 mg 2 pcs
€13.42 €11.18
Description
Interacts with 5-hydroxytryptamine type 1 receptors located in the smooth muscle of the walls of blood vessels of the brain.
Stimulation of 5HT1 receptors leads to selective vasoconstriction in the carotid artery system and reduction of neurogenic inflammation processes, while not having a significant effect on cerebral blood flow. In addition, it is experimentally established that sumatriptan inhibits trigeminal nerve activity.
Preferentially narrows the vessels in the carotid artery system, inhibits the activity of the trigeminal nerve. Contributes to reduction of the severity of pain syndrome in migraine. The clinical effect is observed within 30 minutes.
Pharmacokinetics
Absorption
. When administered orally, sumatriptan is rapidly absorbed from the gastrointestinal tract, reaching a plasma concentration of 70% of Cmax after 45 min. Absolute bioavailability when administered orally due to presystemic metabolism and incomplete absorption is on average 14%.
Distribution
The binding to plasma proteins is 14-21%.
Metabolism
It is biotransformed with the formation of metabolites, the main one being the indoleukasic analogue sumatriptan.
Elevation
T1/2 is 2 h. The main metabolite of sumatriptan is excreted mainly in the urine as free acid or glucuronide conjugate
.
Indications
Indications
Relief of acute migraine attacks, with or without aura.
Pharmacological effect
Pharmacological effect
Interacts with type 1 5-hydroxytryptamine receptors located in the smooth muscles of the walls of blood vessels in the brain.
Stimulation of 5HT1 receptors leads to selective vasoconstriction in the carotid artery system and a decrease in neurogenic inflammation, without significantly affecting cerebral blood flow. In addition, it has been experimentally established that sumatriptan inhibits the activity of the trigeminal nerve.
Selectively narrows blood vessels in the carotid artery system, inhibits the activity of the trigeminal nerve. Helps reduce the severity of pain during migraine. The clinical effect is observed after 30 minutes.
Pharmacokinetics
Suction
When the drug is taken orally, sumatriptan is quickly absorbed from the gastrointestinal tract; after 45 minutes its plasma concentration reaches 70% of Cmax. Absolute bioavailability when taken orally due to first-pass metabolism and incomplete absorption averages 14%.
Distribution
Plasma protein binding is 14-21%.
Metabolism
It is biotransformed to form metabolites, the main of which is the indoleacetic analogue of sumatriptan.
Removal
T1/2 is 2 hours. The main metabolite of sumatriptan is excreted primarily in the urine in the form of a free acid or glucuronide conjugate
Special instructions
Special instructions
When prescribing Amigrenin to patients with newly diagnosed migraine or migraine with atypical symptoms, other potentially dangerous neurological diseases should be excluded. It must be borne in mind that patients with migraine are at risk of developing cerebrovascular complications (including stroke or transient cerebrovascular accident).
The drug should not be prescribed to patients at risk of developing pathology from the cardiovascular system, without prior examination to exclude the disease. The first 2-3 doses of the drug should be carried out under the supervision of a doctor (as spasm of the coronary arteries is possible).
In patients with hypersensitivity to sulfonamides, when taking Amigrenin, allergic reactions may develop, which range from skin manifestations to anaphylactic shock.
If there is no effect on the first dose, the diagnosis should be clarified.
Clinical experience with the drug in patients over 65 years of age is limited (no significant differences in pharmacokinetics are observed compared to younger patients).
Impact on the ability to drive vehicles and operate machinery
During therapy with Amigrenin, drowsiness may develop. Therefore, during the period of use of the drug, patients should be especially careful when driving a car and engaging in other potentially hazardous activities that require a high speed of psychomotor reactions.
Active ingredient
Active ingredient
Sumatriptan
Composition
Composition
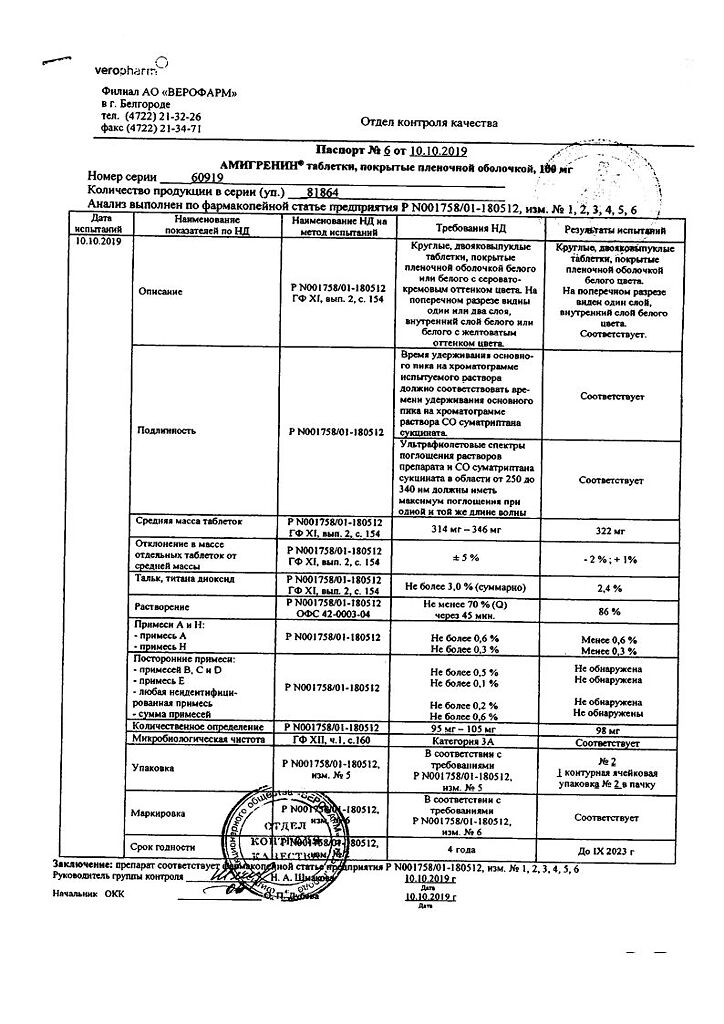
1 tablet contains sumatriptan (in the form of succinate) 100 mg;
Excipients:
microcrystalline cellulose – 32 mg,
lactose (milk sugar) – 123.7 mg,
potato starch – 11.5 mg,
sodium carboxymethyl starch (Primogel) – 9.6 mg,
magnesium stearate – 3.2 mg.
Shell composition:
hypromellose (hydroxypropyl methylcellulose) – 4.18 mg,
povidone (polyvinylpyrrolidone) – 2.8 mg,
macrogol 4000 (polyethylene glycol 4000) – 1.02 mg,
talc – 1.12 mg,
titanium dioxide (E171) – 880 mcg.
Pregnancy
Pregnancy
The drug is contraindicated for use during pregnancy and lactation (breastfeeding).
Use in children
The drug is contraindicated in children and adolescents under 18 years of age.
Contraindications
Contraindications
Individual intolerance to the drug, ischemic heart disease, incl. angina pectoris, hemiplegic, basilar or ophthalmoplegic forms of migraine, occlusive diseases of the peripheral arteries, uncontrolled arterial hypertension, stroke or transient cerebrovascular accident in history, liver failure, pregnancy, breastfeeding, childhood, old age (over 65 years), simultaneous use with ergotamine and its derivatives.
With caution – epilepsy (including any conditions with a reduced epileptic threshold), arterial hypertension (controlled), simultaneous use of MAO inhibitors and a period of up to 14 days after their discontinuation.
Side Effects
Side Effects
Feeling of tingling, warmth, heaviness, pressure in various parts of the body, hyperemia of the skin and mucous membranes; dizziness, fatigue, weakness, drowsiness; angina attack, hypotension, tachycardia, palpitations, transient increase in blood pressure, transient ischemic ECG changes, bradycardia, in isolated cases – Raynaud’s syndrome; feeling of abdominal discomfort, dysphagia, nausea, vomiting, ischemic colitis; visual impairment (diplopia, scotoma, decreased visual acuity); allergic reactions (rash, urticaria, itching, erythema, anaphylaxis); changes in liver function tests; myalgia.
Interaction
Interaction
The simultaneous use of drugs containing ergot alkaloids, lithium preparations, neuronal serotonin reuptake inhibitors, and MAO inhibitors is prohibited.
When taken simultaneously with ergotamine, prolonged vasospasm was observed. Amigrenin can be prescribed no earlier than 24 hours after taking medications containing ergotamine.
Overdose
Overdose
Symptoms: increased side effects.
Treatment: in case of overdose, the patient should be observed for 10 hours, providing symptomatic therapy as necessary.
Storage conditions
Storage conditions
In a dry place, protected from light
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Veropharm LLC, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place |
| Manufacturer | Veropharm AO, Russia |
| Medication form | pills |
| Brand | Veropharm AO |
Other forms…
Related products
Buy Amigrenin, 100 mg 2 pcs with delivery to USA, UK, Europe and over 120 other countries.