No products in the cart.
Description
Pharmacodynamics
Antiviral drug – synthetic analog of thymidine nucleoside.In infected cells containing viral thymidine kinase, phosphorylation and conversion to acyclovir monophosphate occurs. Under the influence of guanylate cyclase acyclovir monophosphate is converted into diphosphate and under the influence of several cellular enzymes into triphosphate.
High selectivity and low toxicity for humans is conditioned by the absence of required enzyme for acyclovir triphosphate formation in intact cells of macroorganism.
Acyclovir triphosphate “integrates” into DNA synthesized by virus and blocks virus multiplication. Specificity and very high selectivity of action are also due to its predominant accumulation in cells affected by the herpes virus. It is highly active against Herpes simplex virus types 1 and 2; Varicella zoster virus and shingles virus; Epstein-Barr virus. Moderately active against cytomegaloviruses.
Pharmacokinetics
When used on intact skin: absorption is minimal; not detected in blood and urine. On affected skin:absorption is moderate; in patients with normal renal function the serum concentration is up to 0.28 µg/ml, in patients with chronic renal failure (CRF) up to 0.78 µg/ml. Excreted by the kidneys (up to 9.4% of the daily dose).
Indications
Indications
Colds, Skin itch, Herpes
– Skin infections caused by Herpes simplex virus types 1 and 2,
– genital herpes,
– shingles,
– varicella.
Active ingredient
Active ingredient
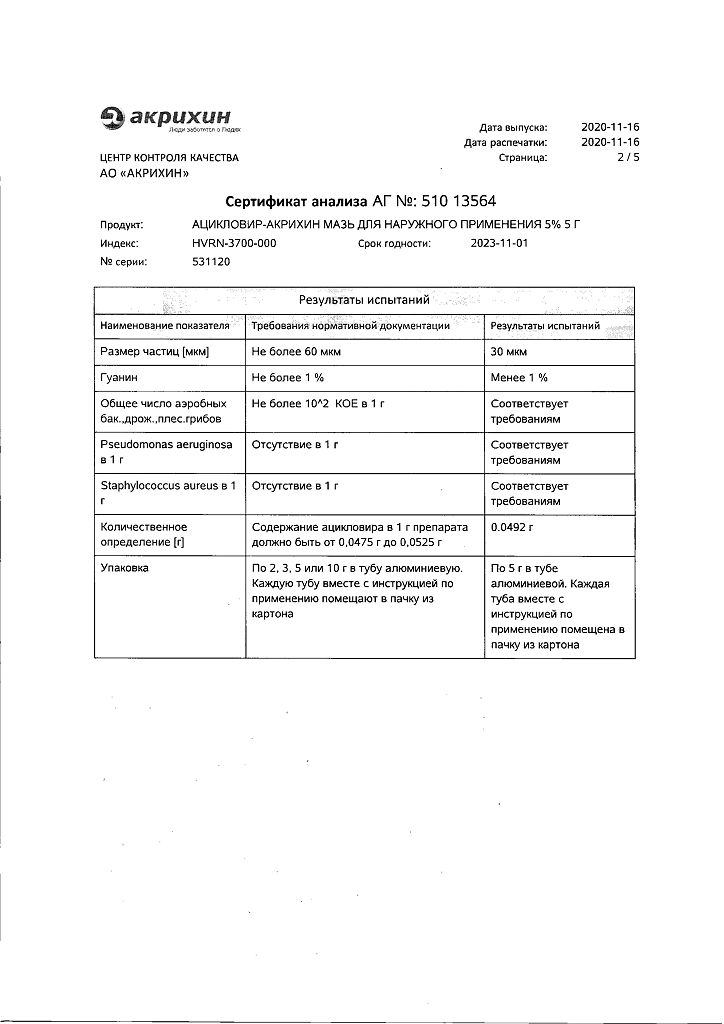
Acyclovir
Composition
Composition
100 g ointment contains:
active substance:
acyclovir in terms of 100% substance – 5 g;
auxiliary substances:
Propylene glycol – 40 g,
Vaseline – 12.5 g,
Vaseline oil – 7.5 g,
emulsion wax – 5 g,
macrogol (polyethylene oxide 1500)-1 g,
purified water up to 100 g.
How to take, the dosage
How to take, the dosage
Outpatiently.
The product is applied with clean hands or with a cotton swab 5 times a day (every 4 hours) in a thin layer on the affected and adjacent skin areas.
The therapy should be continued until a crust has formed on the blisters or until they are completely healed.
The duration of therapy is on average 5 days, maximum 10 days.
If there is no effect it is necessary to consult a doctor.
Interaction
Interaction
No interaction with other drugs was found during external use.
Strengthening of the effect is observed with the simultaneous administration of immunostimulants.
Special Instructions
Special Instructions
To achieve maximum therapeutic effect it is necessary to use the drug as soon as possible (at the first signs of the disease: burning, itching, tingling, a feeling of tension and redness).
The ointment is not recommended to apply to the mucous membranes of the mouth and eyes because pronounced local inflammation may develop.
When treating genital herpes sexual intercourse should be avoided or condoms should be used, because the use of acyclovir does not prevent transmission to partners.
Contraindications
Contraindications
Hypersensitivity to acyclovir and other components of the drug.
With caution – pregnancy, lactation, dehydration, renal failure.
Side effects
Side effects
– Hyperemia, dryness, peeling of the skin;
– burning, inflammation in contact with mucous membranes.
Allergic dermatitis may develop.
Overdose
Overdose
Symptoms (if accidentally swallowed):
– headache,
– neurological disturbances,
– shortness of breath,
– nausea,
– vomiting,
– diarrhea,
– impaired renal function,
– lethargy,
– convulsions,
– coma.
Treatment:
– maintenance of vital functions,
– hemodialysis.
.
Pregnancy use
Pregnancy use
The use of the drug is indicated only when the expected benefit to the mother exceeds the potential risk to the fetus.
The cessation of breastfeeding should be considered during treatment.
Similarities
Similarities
Zovirax, Vivorax, Acyclovir-Acri, Acyclovir, Herperax, Acyclovir Belupo, Acyclovir Sandoz
Additional information
| Weight | 0.015 kg |
|---|---|
| Shelf life | 3 years. |
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Akrihin HFC JSC, Russia |
| Medication form | topical ointment |
| Brand | Akrihin HFC JSC |
Other forms…
Related products
Buy Aciclovir-Acrihin, 5% ointment 5 g with delivery to USA, UK, Europe and over 120 other countries.