No products in the cart.
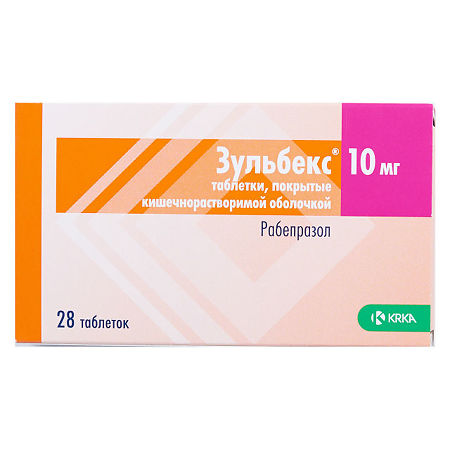
Zulbecs, 10 mg 28 pcs
€16.09 €13.95
EAN: 3838989582166
SKU: 216294
Categories: Medicine, Stomach, intestines, liver, Ulcer and gastritis
Description
Zulbecs are antiulcer.
Pharmacodynamics
Rabeprazole belongs to the class of antisecretory drugs, substituted benzimidazoles, which have no cholinolytic or antihistamine (H2) properties, but inhibit gastric secretion by inhibiting the enzyme H+/K+-ATPase (proton pump). The effect of the drug is dose-dependent and results in suppression of basal and stimulated gastric hydrochloric acid secretion, regardless of stimulating factors.
Animal studies have shown that rabeprazole rapidly disappears from plasma and gastric mucosa. As a weak base, rabeprazole is rapidly absorbed in all dosages and accumulates in the acidic environment of the parietal cells of the stomach. Rabeprazole is converted to the sulfenamide form by protonation and then interacts with available proton pump cysteine molecules.
Antisecretory action: after oral administration of 20 mg of rabeprazole, the antisecretory effect begins to develop within 1 h, reaching a maximum after 2-4 h. Suppression of basal and food stimulated secretion of hydrochloric acid in the stomach 23 h after the first dose of rabeprazole is 69% and 82%, respectively, and lasts up to 48 h.
The inhibitory effect of rabeprazole on hydrochloric acid secretion increases slightly with repeated doses, reaching an equilibrium state after 3 days. After discontinuation of the drug, gastric secretory activity is restored after 2-3 days.
In vitro it was found that rabeprazole has a bactericidal effect on Helicobacter pylori.Eradication of H. pylori with rabeprazole and antimicrobials leads to a high degree of healing of mucosal lesions.
The results of clinical studies show that administration of rabeprazole 20 mg 2 times a day in combination with two antibiotics, e.g. clarithromycin and amoxicillin or clarithromycin and metronidazole for 1 week allows to reach the eradication of H. pylori over 80% in patients with gastroduodenal ulcers.
When selecting the appropriate combination for H. pylori eradication, approved treatment standards should be followed.
In patients with persistent infection (in the presence of initially susceptible strains of microorganisms) the possibility of development of secondary resistance to antibacterial drugs must be considered when choosing a dosing regimen.
Impact on serum gastrin: in clinical studies patients received rabeprazole in doses of 10 or 20 mg once daily for up to 43 months.
The serum concentration of gastrin increased in the first 2-8 weeks of therapy, reflecting the suppressive effect on hydrochloric acid secretion, and then remained stable with continuation of therapy. Gastrin concentrations usually returned to baseline values within 1-2 weeks after therapy withdrawal.
Biopsy specimens from the antral part and fundus of the stomach obtained from more than 500 patients taking rabeprazole or comparative treatment for up to 8 weeks showed no changes in ECL cellular and histological structure, gastritis grade, frequency of atrophic gastritis, intestinal metaplasia, or prevalence of H. pylori in more than 250 patients followed for 36 months of therapy showed no significant changes in baseline conditions.
Other effects: systemic effects of rabeprazole on the CNS, cardiovascular and respiratory systems have not been identified to date.
Rabeprazole administered orally at a dose of 20 mg for 2 weeks had no effect on thyroid function, carbohydrate metabolism or circulating concentrations of parathormone, cortisol, estrogen, testosterone, prolactin, cholecystokinin, secretin, glucagon, FSH, LH, renin, aldosterone or STH.
Clinical studies have shown that rabeprazole has no clinically significant interaction with amoxicillin and no adverse effect on plasma concentrations of amoxicillin or clarithromycin when these drugs are used simultaneously to eradicate H. pylori in the upper GI tract.
Pharmacokinetics
Absorption: The drug Zulbecs® is an enteric-soluble (gastric-stable) tablet coated with rabeprazole.
This form is due to the instability of rabeprazole in an acidic environment. Therefore, absorption of rabeprazole begins only after the tablet leaves the stomach. Absorption is rapid; Cmax of rabeprazole in plasma is reached approximately 3.5 h after an oral dose of 20 mg. Cmax and AUC are linear in the dose range from 10 mg to 40 mg.
The absolute bioavailability of an oral dose of 20 mg (compared with intravenous administration) is approximately 52%, largely due to presystemic metabolism.
The bioavailability does not appear to increase with repeated administration. In healthy subjects, the T1/2 from plasma is approximately 1 h (0.7 to 1.5 h) and total clearance is 283±98 ml/min. There is no clinically significant interaction associated with food intake. Neither food nor time of drug intake affects absorption of rabeprazole.
Distribution: In humans rabeprazole is approximately 97% bound to plasma proteins.
Metabolism and excretion: Rabeprazole, like other representatives of the class of proton pump inhibitors, is metabolized in the liver, with the participation of cytochrome P450 (CYP450). In vitro studies with human hepatic microsomes have shown that rabeprazole is metabolized by CYP450 isoenzymes (CYP2C19 and CYP3A4).
In these studies, rabeprazole did not inhibit or stimulate CYP3A4 at the expected plasma concentrations in humans. These results suggest that no interaction is expected between rabeprazole and cyclosporine.
In humans, the major metabolites detected in plasma are thioether (M1) and carboxylic acid (M6), and the sulfonic metabolite (M2), desmethylthioether (M4), and the mercapturic acid conjugate (M5) are determined in lower amounts. Only the desmethyl metabolite (M3) has little antisecretory activity, but it is not detected in plasma.
After a single oral dose of 20 mg of 14C-labeled rabeprazole, unchanged rabeprazole is not excreted by the kidneys. Approximately 90% of the administered dose is excreted by the kidneys as two metabolites: mercapturic acid conjugate (M5) and carboxylic acid (M6), and as two unknown metabolites. The rest of the administered drug is found in the intestinal contents.
Gender: Adjusted for height and body weight, there were no sex differences in the pharmacokinetic parameters of rabeprazole in the 20 mg dose.
Renal dysfunction: in patients with renal insufficiency requiring hemodialysis (cLcreatinine less than 5 ml/min/1.73 m2) the distribution of rabeprazole was similar to that in healthy volunteers. The AUC and Cmax in such patients were approximately 35% lower than those in healthy volunteers.
The mean T1/2 of rabeprazole was 0.82 h in healthy volunteers, 0.95 h in patients on hemodialysis and 3.6 h after hemodialysis. The creatinine Cl of rabeprazole in patients with impaired renal function requiring maintenance hemodialysis was approximately 2 times higher than in healthy volunteers.
Hepatic impairment: after a single dose of 20 mg of rabeprazole in patients with mild to moderate liver disease, the AUC was 2-fold increased and the T1/2 of rabeprazole was 2-3-fold increased compared to healthy volunteers.
However, after a daily oral dose of 20 mg for 7 days, the AUC increased only 1.5-fold and the Cmax increased only 1.2-fold. The T1/2 of rabeprazole in patients with impaired liver function was 12.3 h, compared with 2.1 h in healthy volunteers. The pharmacodynamic response (gastric pH control) in the two groups was clinically comparable.
Elderly patients: The excretion of rabeprazole is slightly reduced in elderly patients. After using rabeprazole for 7 days at a daily dose of 20 mg, the AUC increased approximately 2-fold and the Cmax increased by 60% , the T1/2 was increased by 30% compared to healthy young volunteers. No evidence of rabeprazole accumulation was noted.
The CYP2C19 polymorphism: After oral administration of rabeprazole at a dose of 20 mg, AUC and T1/2 were approximately 1.9 and 1.6 times higher than the corresponding parameters in those with active metabolism, while Cmax was only increased by 40% in people with delayedCYP2C19-metabolism.
Indications
Indications
Peptic ulcer of the stomach and duodenum in the acute stage;
Gastroesophageal reflux disease (GERD): erosive reflux esophagitis (treatment), symptomatic treatment of GERD, including long-term maintenance therapy;
Zollinger-Ellison syndrome;
As part of complex therapy: eradication of Helicobacter pylori in patients with gastric and duodenal ulcers or chronic gastritis;
Treatment and prevention of relapse of peptic ulcer associated with Helicobacter pylori.
Pharmacological effect
Pharmacological effect
Zulbex – antiulcer.
Pharmacodynamics
Rabeprazole belongs to the class of antisecretory drugs, the substituted benzimidazoles, which do not have anticholinergic or antihistamine (H2) properties, but suppress gastric secretion by inhibiting the enzyme H+/K+-ATPase (proton pump). The effect of the drug depends on the dose and leads to the suppression of basal and stimulated secretion of hydrochloric acid in the stomach, regardless of stimulating factors.
Animal studies have shown that rabeprazole rapidly disappears from the plasma and gastric mucosa. Being a weak base, rabeprazole is rapidly absorbed in all dosages and accumulates in the acidic environment of the parietal cells of the stomach. Rabeprazole is converted to the sulfenamide form by protonation and then interacts with available cysteine molecules of the proton pump.
Antisecretory effect: after oral administration of 20 mg of rabeprazole, the antisecretory effect begins to develop within 1 hour, reaching a maximum after 2–4 hours. Suppression of basal and food-stimulated secretion of hydrochloric acid in the stomach 23 hours after taking the first dose of rabeprazole is 69% and 82%, respectively, and lasts up to 48 hours.
The inhibitory effect of rabeprazole on hydrochloric acid secretion increases slightly with repeated doses, reaching a steady state after 3 days. After discontinuation of the drug, the secretory activity of the stomach is restored within 2–3 days.
In vitro, it was found that rabeprazole has a bactericidal effect on Helicobacter pylori. Eradication of H. pylori with rabeprazole and antimicrobial drugs leads to a high degree of healing of mucosal lesions.
According to the results of clinical studies, it was found that taking 20 mg of rabeprazole 2 times a day in combination with two antibiotics, for example clarithromycin and amoxicillin or clarithromycin and metronidazole for 1 week, can achieve a level of H. pylori eradication of more than 80% in patients with gastroduodenal ulcers.
Established treatment standards should be used to select the appropriate combination for H. pylori eradication.
In patients with persistent infection (in the presence of initially sensitive strains of microorganisms), it is necessary to take into account the possibility of developing secondary resistance to antibacterial drugs when choosing a dosage regimen.
Effect on serum gastrin: in clinical studies, patients received rabeprazole at a dose of 10 or 20 mg once daily for up to 43 months.
Serum gastrin concentrations increased during the first 2–8 weeks of dosing, reflecting a suppressive effect on hydrochloric acid secretion, and then remained stable with continued therapy. Gastrin concentrations returned to baseline values usually within 1–2 weeks after discontinuation of therapy.
Biopsies from the antrum and fundus of the stomach obtained from more than 500 patients treated with rabeprazole or comparator treatment for up to 8 weeks revealed no changes in ECL cellularity, histology, grade of gastritis, incidence of atrophic gastritis, intestinal metaplasia, or prevalence of H. pylori infection in more than 250 patients followed over 36 months of therapy. there were no significant changes in pre-existing conditions.
Other effects: the systemic effect of rabeprazole on the central nervous system, cardiovascular and respiratory systems has not been identified to date.
Rabeprazole, administered orally at a dose of 20 mg for 2 weeks, had no effect on thyroid function, carbohydrate metabolism, or circulating concentrations of parathyroid hormone, cortisol, estrogens, testosterone, prolactin, cholecystokinin, secretin, glucagon, FSH, LH, renin, aldosterone, or growth hormone.
Clinical studies have shown that rabeprazole does not interact clinically with amoxicillin and does not have a negative effect on the concentration of amoxicillin or clarithromycin in the blood plasma when these drugs are used simultaneously for the eradication of H. pylori in the upper gastrointestinal tract.
Pharmacokinetics
Absorption: Zulbex® is a rabeprazole tablet coated with an enteric (stomach-resistant) coating.
This form is due to the instability of rabeprazole in an acidic environment. Therefore, absorption of rabeprazole begins only after the tablet leaves the stomach. Absorption is fast; Cmax of rabeprazole in blood plasma is reached approximately 3.5 hours after oral administration of a dose of 20 mg. Cmax and AUC are linear in the dose range from 10 mg to 40 mg.
The absolute bioavailability of an oral dose of 20 mg (compared to intravenous administration) is approximately 52%, largely due to first-pass metabolism.
Bioavailability does not appear to increase with repeated use. In healthy people, T1/2 from blood plasma is approximately 1 hour (from 0.7 to 1.5 hours), and the total clearance is 283 ± 98 ml/min. There is no clinically significant interaction associated with food intake. Neither food nor the time of taking the drug affects the absorption of rabeprazole.
Distribution: In humans, rabeprazole is approximately 97% bound to plasma proteins.
Metabolism and elimination: rabeprazole, like other members of the class of proton pump inhibitors, is metabolized in the liver with the participation of cytochrome P450 (CYP450). In vitro studies with human liver microsomes have shown that rabeprazole is metabolized by CYP450 isoenzymes (CYP2C19 and CYP3A4).
In these studies, rabeprazole at expected plasma concentrations in humans did not suppress or stimulate CYP3A4. These results indicate that no interaction is expected between rabeprazole and cyclosporine.
In humans, the main metabolites found in plasma are the thioester (M1) and carboxylic acid (M6), with the sulfonic metabolite (M2), desmethylthioester (M4) and mercapturic acid conjugate (M5) detected in smaller quantities. Only the desmethyl metabolite (M3) has little antisecretory activity, but it is not detected in plasma.
After a single oral dose of 20 mg of 14C-labeled rabeprazole, unchanged rabeprazole is not excreted by the kidneys. Approximately 90% of the dose taken is excreted by the kidneys in the form of two metabolites: a conjugate with mercapturic acid (M5) and carboxylic acid (M6), as well as in the form of two unknown metabolites. The rest of the administered drug is found in the intestinal contents.
Gender: After adjusting for height and body weight, there were no sex differences in the pharmacokinetic parameters of rabeprazole at a dose of 20 mg.
Impaired renal function: in patients with renal failure requiring hemodialysis (Cl creatinine less than 5 ml/min/1.73 m2), the distribution of rabeprazole was similar to its distribution in healthy volunteers. AUC and Cmax in such patients were approximately 35% lower than the corresponding values in healthy volunteers.
The average T1/2 of rabeprazole was 0.82 hours in healthy volunteers, 0.95 hours in patients undergoing hemodialysis and 3.6 hours after hemodialysis. Rabeprazole creatinine Cl in patients with impaired renal function requiring maintenance hemodialysis was approximately 2 times higher than in healthy volunteers.
Liver dysfunction: after a single dose of rabeprazole 20 mg in patients with mild or moderate liver disease, the AUC increased by 2 times and the T1/2 of rabeprazole increased by 2-3 times compared to healthy volunteers.
However, after daily oral administration of a dose of 20 mg for 7 days, AUC increased only 1.5 times, and Cmax increased only 1.2 times. T1/2 of rabeprazole in patients with impaired liver function was 12.3 hours compared to 2.1 hours in healthy volunteers. The pharmacodynamic response (control of gastric pH) in the two groups was clinically comparable.
Elderly patients: In elderly patients, the elimination of rabeprazole is slightly reduced. After using rabeprazole for 7 days at a daily dose of 20 mg, AUC increased approximately 2 times, Cmax increased by 60%, T1/2 was increased by 30% compared to healthy young volunteers. There were no signs of accumulation of rabeprazole.
CYP2C19 polymorphism: after oral administration of rabeprazole at a dose of 20 mg in people with slow CYP2C19 metabolism, AUC and T1/2 were approximately 1.9 and 1.6 times higher than the corresponding parameters in people with active metabolism, while Cmax increased only by 40%.
Special instructions
Special instructions
A decrease in the severity of symptoms during therapy with Zulbex® does not exclude the presence of malignant neoplasms in the stomach or esophagus, therefore, before starting therapy, it is necessary to conduct an examination to exclude gastrointestinal neoplasms.
Patients receiving long-term therapy with Zulbex® (especially more than one year) should undergo regular examination.
The risk of cross-reactions with other proton pump inhibitors or with substituted benzimidazoles cannot be excluded.
The patient must be warned that the tablets must be swallowed whole, without chewing or breaking.
The drug Zulbex® is not recommended for use in children, because There is no experience with the use of the drug in this group of patients.
There are post-marketing reports of the development of blood dyscrasias (cases of thrombocytopenia and neutropenia) with the use of rabeprazole. In most cases, when it was not possible to find out alternative causes of these conditions, they did not cause complications and resolved after rabeprazole was discontinued.
During the use of the drug Zulbex®, changes in the activity of liver enzymes are possible, which occurs after discontinuation of the drug.
In a study in patients with mild to moderate hepatic impairment, there were no significant safety concerns with rabeprazole compared with age- and gender-matched healthy controls. Due to the lack of clinical data on the use of rabeprazole in patients with severe liver dysfunction, caution is recommended when using Zulbex® in this group of patients.
Impact on the ability to drive vehicles and other complex mechanisms:
Based on the properties of rabeprazole, it is unlikely that Zulbex® can impair the ability to drive vehicles or affect the work with technical devices.
If side effects develop (drowsiness, dizziness, confusion), you should stop driving and work that requires increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Rabeprazole
Composition
Composition
1 tablet contains:
Active substance:
rabeprazole sodium 10 mg;
Excipients:
mannitol (E421);
magnesium oxide light;
hyprolose;
low-substituted hyprolose;
magnesium stearate.
Pregnancy
Pregnancy
Pregnancy
There are no data on the safety of rabeprazole during pregnancy in humans. Reproductive studies in rats and rabbits showed no evidence of impaired fertility or harmful effects of rabeprazole on the fetus.
Zulbex is not used during pregnancy.
Lactation
It is unknown whether rabeprazole is secreted into human milk, but is secreted into rat milk. Studies have not been conducted in women during lactation.
If it is necessary to use the drug Zulbex during lactation, breastfeeding should be stopped.
Contraindications
Contraindications
Hypersensitivity to the active substance or auxiliary components of the drug, pregnancy, breastfeeding, childhood (no experience of use).
With caution
Severe renal failure.
Side Effects
Side Effects
From the hematopoietic system: rarely – neutropenia, leukopenia, thrombocytopenia, leukocytosis.
From the immune system: rarely – hypersensitivity reactions.
Metabolic and nutritional disorders: rarely – anorexia, weight gain; very rarely – hyponatremia.
From the nervous system: often – headache, dizziness, insomnia; infrequently – drowsiness, nervousness; rarely – depression; very rarely – confusion.
From the senses: rarely – visual disturbances.
From the cardiovascular system: very rarely – peripheral edema.
From the respiratory system: often – cough, pharyngitis, rhinitis; infrequently – bronchitis, sinusitis.
From the digestive system: often – diarrhea, vomiting, nausea, abdominal pain, constipation, flatulence; uncommon – dyspepsia, dry oral mucosa, belching; rarely – gastritis, stomatitis, taste changes, hepatitis, jaundice, hepatic encephalopathy.
From the skin: infrequently – rash, erythema; rarely – itching, sweating, bullous rash; very rarely – erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome.
From the musculoskeletal system: often – nonspecific pain, back pain; uncommon – myalgia, calf muscle cramps, arthralgia.
From the urinary system: infrequently – urinary tract infections; rarely – interstitial nephritis.
From the reproductive system: very rarely – gynecomastia.
Laboratory indicators: infrequently – increased activity of liver enzymes.
Other: often – asthenia, influenza-like illness.
Interaction
Interaction
Rabeprazole causes persistent and long-term suppression of hydrochloric acid secretion in the stomach. Interactions may occur with drugs whose absorption is dependent on pH values. Concomitant use of rabeprazole with ketoconazole or itraconazole may lead to a significant decrease in their plasma concentrations, which may require dose adjustment of these drugs.
Proton pump inhibitors, including rabeprazole, should not be used concomitantly with atazanavir.
Rabeprazole does not have clinically significant interactions with amoxicillin and other drugs metabolized by enzymes of the cytochrome CYP450 system, such as warfarin, phenytoin, theophylline and diazepam.
Rabeprazole slows down the elimination of some drugs metabolized in the liver by microsomal oxidation (diazepam, phenytoin, indirect anticoagulants).
Plasma concentrations of rabeprazole and the active metabolite clarithromycin increase by 24% and 50%, respectively, when administered concomitantly. Reduces the concentration of ketoconazole by 33%, digoxin by 22%.
Overdose
Overdose
Current experience with intentional or accidental overdose with rabeprazole is limited. The maximum prescribed amount of the drug taken did not exceed 60 mg twice a day or 160 mg once a day.
The effects were insignificant and corresponded to the known range of adverse reactions, which resolved spontaneously without any additional medical intervention. A specific antidote is unknown. Dialysis is ineffective.
Treatment: symptomatic.
Storage conditions
Storage conditions
At a temperature not exceeding 30 °C.
Shelf life
Shelf life
2 years.
Manufacturer
Manufacturer
KRKA dd Novo Mesto, Slovenia
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C. |
| Manufacturer | KRKA dd Novo mesto, Slovenia |
| Medication form | enteric soluble tablets |
| Brand | KRKA dd Novo mesto |
Other forms…
Related products
Buy Zulbecs, 10 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.