No products in the cart.
Xarelto, 10 mg 100 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
A selective direct inhibitor of factor Xa for oral administration. Activation of factor X to form factor Xa via intrinsic and extrinsic pathways plays a central role in the coagulation cascade.
Rivaroxaban has a dose-dependent effect on prothrombin time and is highly correlated with plasma concentrations when analyzed with the Neoplastin kit (results will differ when using other reagents).
Rivaroxaban also dose-dependently increases the ABTB and Heptest result, but these parameters are not recommended for assessing the pharmacodynamic effects of rivaroxaban.
In healthy men and women over 50 years of age, QT interval prolongation was not observed with rivaroxaban.
Pharmacokinetics
- absorption
The absolute bioavailability of rivaroxaban after a 10 mg dose is high (80-100%). Rivaroxaban is rapidly absorbed; C max is reached 2-4 h after tablet ingestion.
No change in AUC and C max was noted when rivaroxaban 10 mg was taken with food. Rivaroxaban at a dose of 10 mg may be administered for administration with meals or independently of meals.
The pharmacokinetics of rivaroxaban are characterized by moderate individual variability; individual variability (variation factor) is 30% to 40%, except for the day of surgery and the following day, when variability in exposure is high (70%).
- Distribution
In humans, most of rivaroxaban (92-95%) binds to plasma proteins, with serum albumin being the major binding component. V d is moderate, V ss is approximately 50 L.
- Metabolism
Rivaroxaban is metabolized via CYP3A4, CYP2J2 isoenzymes, and by mechanisms independent of the cytochrome system. The main sites of biotransformation are oxidation of the morpholine group and hydrolysis of the amide bonds.
According to the data obtained in vitro rivaroxaban is a substrate for P-gp (P-glycoprotein) and BPGr (breast cancer resistance protein) carrier proteins.
Unchanged rivaroxaban is the only active compound in human plasma; no significant or active circulating metabolites have been detected in plasma. Rivaroxaban, which has a systemic clearance of approximately 10 L/h, may be classified as a low clearance drug.
- Elimation
In plasma excretion of rivaroxaban, the final T 1/2 is 5 to 9 h in young patients.
When administered orally, approximately 2/3 of the prescribed dose of rivaroxaban is metabolized and subsequently excreted in equal parts in the urine and feces. The remaining one third of the dose is excreted via direct renal excretion unchanged, mainly due to active renal secretion.
Pharmacokinetics in special clinical cases
- In elderly patients (>65 years)
Plasma concentrations of rivaroxaban are higher than in younger patients, with average AUC values approximately 1.5 times higher than those in younger patients, primarily due to seemingly lower total and renal clearance. In plasma excretion of rivaroxaban, the final T 1/2 in elderly patients is 11 to 13 h.
- In men and women
Clinically significant differences in pharmacokinetics have not been found.
Too little or too much body weight (less than 50 kg and more than 120 kg)
Only slightly affects the plasma concentration of rivaroxaban (less than 25% difference).
- In children
There are no data on pharmacokinetics in children.
- Differences in pharmacokinetics and pharmacodynamics in patients of Caucasian, African-American, Hispanic, Japanese, or Chinese ethnicity
There were no clinically significant differences observed.
- In patients with liver disease
The effect of hepatic impairment on rivaroxaban pharmacokinetics was studied in patients graded according to the Child-Pugh classification (according to standard procedures in clinical trials). The Child-Pugh classification allows to assess the prognosis of chronic liver diseases, mainly cirrhosis.
In patients scheduled for anticoagulant therapy, a particularly critical point of impaired liver function is the decreased synthesis of clotting factors in the liver. Because this figure corresponds to only one of the five clinical/biochemical criteria that make up the Child-Pugh classification, the risk of bleeding does not correlate clearly with this classification. Treatment of such patients with anticoagulants should be decided regardless of the Child-Pugh classification.
Rivaroxaban is contraindicated in patients with liver disease with coagulopathy that is clinically significant bleeding risk. Pharmacokinetics of rivaroxaban in patients with mild hepatic impairment (class A according to Child-Pugh classification) did not significantly differ from those in control group of healthy subjects (on average there was 1.2 times increase of AUC of rivaroxaban). There were no significant differences in pharmacodynamic properties between the groups.
In patients with cirrhosis and moderate hepatic impairment (Child-Pugh class B), the mean AUC of rivaroxaban was significantly elevated (2.3-fold) compared with healthy volunteers due to significantly reduced drug clearance, indicating severe liver disease. Suppression of factor Xa activity was more pronounced (2.6-fold) than in healthy volunteers.
The prothrombin time was also 2.1 times that of healthy volunteers. Prothrombin time measurement assesses the external coagulation pathway, which includes clotting factors VII, X, V, II and I, which are synthesized in the liver. Patients with moderate hepatic insufficiency are more sensitive to rivaroxaban, which is a consequence of the closer relationship between pharmacodynamic effects and pharmacokinetic parameters, especially between concentration and prothrombin time.
No data are available for patients with Child-Pugh class C hepatic insufficiency.
- In patients with renal impairment
An increase in plasma concentrations of rivaroxaban was observed that was inversely proportional to decreased renal function as assessed by creatinine clearance .
In patients with mild (KC 80-50 ml/min), moderate (KC < 50-30 ml/min), or severe (KC < 30-15 ml/min) renal impairment, 1.4-, 1.5-, and 1.6-fold increases in plasma concentrations of rivaroxaban were observed compared with healthy volunteers, respectively.
The corresponding increase in pharmacodynamic effects was more pronounced. In patients with mild, moderate and severe renal impairment total suppression of factor Xa activity was increased by 1.5, 1.9 and 2 times compared to healthy volunteers; prothrombin time due to factor Xa activity was also increased by 1.3, 2.2 and 2.4 times, respectively.
The data on the use of rivaroxaban in patients with a CKR of 30-15 ml/min are limited, and therefore caution should be exercised when using the drug in this patient population. There are no data on the use of rivaroxaban in patients with IQ < 15 mL/min, therefore, caution is not recommended for this patient population.
Indications
Indications
Prevention of venous thromboembolism in patients undergoing extensive orthopedic surgery on the lower extremities.
Active ingredient
Active ingredient
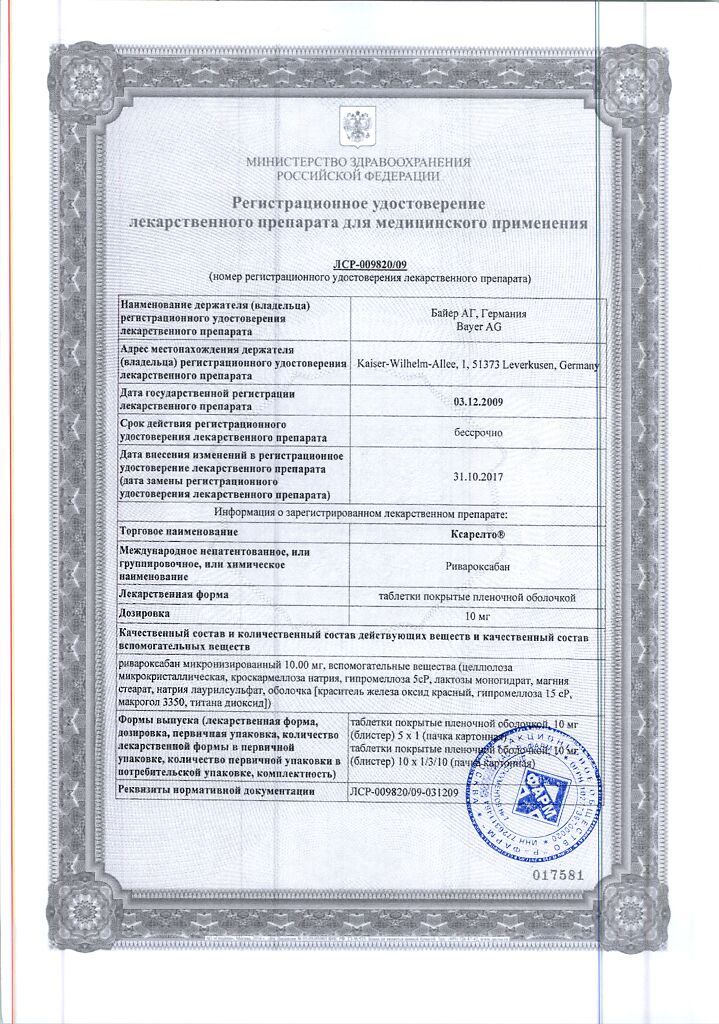
Composition
Composition
1 tabletcarivaroxaban (micronized)10 mg
Supplementary substances:
Microcrystalline cellulose – 40 mg,
croscarmellose sodium – 3 mg,
hypromellose 5cP – 3 mg,
lactose monohydrate – 27.9 mg,
magnesium stearate – 600 µg,
sodium lauryl sulfate – 500 µg.
Shell contents:
Red iron oxide dye – 15 µg,
Hypromellose 15cP – 1.5 mg,
macrogol 3350 – 500 µg,
titanium dioxide – 485 µg.
How to take, the dosage
How to take, the dosage
For the prevention of VTE in major orthopedic surgeries
Preventive therapy is indicated for oral administration of 10 mg once daily. The duration of treatment is determined by the type of orthopedic intervention.
The duration of treatment: 5 weeks after major hip surgery; 2 weeks after major knee surgery.
Rivaroxaban can be taken regardless of meals.
The initial dose should be taken 6-10 h after surgery if hemostasis is achieved.
If a dose is missed, the patient should take rivaroxaban immediately and continue treatment the next day at 1 tablet/day as before.
Transfer patients from vitamin K antagonists (VKAs) to Xarelto
If MHO ⤠3, AVK treatment should be discontinued and Xarelto should be started. When transferring patients from AVC to Xarelto, MHO values will falsely increase after taking Xarelto. Therefore, MHO values should not be used to monitor the anticoagulant effect of Xarelto.
Transition from Xarelto to vitamin K antagonists (VKAs)
There is a possibility of insufficient anticoagulant effect when patients are transferred from Xarelto to VKAs. During the transition period associated with transfer to another anticoagulant drug, it is necessary to ensure a continuous and sufficient anticoagulant effect. Keep in mind that Xarelto may contribute to an increase in MHO.
When converting a patient from Xarelto to AVC, both drugs should be given simultaneously until MHO reaches a value ⥠2. The standard dose of AVC should be used for the first two days of the transition period, and the MHO value should guide thereafter. During coadministration of Xarelto and AVC, MHO should be determined no earlier than 24 h (after the previous but before the next dose of Xarelto). After withdrawal of Xarelto, MHO determination with sufficient reliability is possible 24 hours after the last dose of the drug.
Transition from parenteral anticoagulants to Xarelto
. When transferring a patient from parenteral anticoagulants to Xarelto, Xarelto should be started 0-2 h before the intended administration of the next dose of parenteral drug (e.g. low molecular weight heparin) or during withdrawal of the long-term used parenteral drug (e.g. intravenous infusion of unfractionated heparin).
Transition from Xarelto to parenteral anticoagulants
The first dose of Xarelto should be stopped and the first dose of parenteral anticoagulant administered at the time the next dose of Xarelto should be taken.
The use in selected groups of patients
There is no need to adjust the dose according to the patient’s age (older than 65 years), sex, body weight or ethnicity.
In liver disease
Rivaroxaban is contraindicated in patients with liver disease accompanied by coagulopathy, which causes a clinically significant risk of bleeding. Patients with other liver diseases do not require dose changes. The limited clinical data available in patients with moderate hepatic impairment (Child-Pugh class B) indicate a significant increase in the pharmacological activity of the drug. No clinical data are available for patients with severe hepatic impairment (Child-Pugh class C).
In the administration of rivaroxaban to patients with mild (CKR 80-50 ml/min) or moderate (CKR < 50-30 ml/min) renal impairment, no dose reduction is required.
In renal disease
Limited clinical data available in patients with severe renal impairment (CK < 30-15 ml/min) show a significant increase in rivaroxaban concentrations in these patients. Rivaroxaban should be used with caution to treat this category of patients.
The use of rivaroxaban is not recommended in patients with CK < 15 ml/min.
Interaction
Interaction
The concomitant use of rivaroxaban and strong CYP3A4 and P-glycoprotein isoenzyme inhibitors may lead to decreased renal and hepatic clearance and thereby significantly increase the AUC of rivaroxaban.
The combined use of rivaroxaban and the azole-type antifungal drug ketoconazole (400 mg once daily), which is a strong inhibitor of CYP3A4 and P-glycoprotein, resulted in a 2.6-fold increase in the mean equilibrium AUC of rivaroxaban and 1.7-fold increase in the mean Cmax of rivaroxaban, accompanied by a significant increase in the pharmacodynamic effects of the drug.
Concomitant use of rivaroxaban and the HIV protease inhibitor ritonavir (600 mg 2 times/day), which is a strong CYP3A4 and P-glycoprotein inhibitor, resulted in a 2.5-fold increase in the mean equilibrium AUC of rivaroxaban and 1.6-fold increase in the mean Cmax of rivaroxaban, accompanied by a significant increase in the pharmacodynamic effects of the drug. Therefore, caution should be exercised when using rivaroxaban in patients concomitantly receiving systemic azole antifungals or HIV protease inhibitors.
Clarithromycin (500 mg 2 times/day), which is a potent CYP3A4 inhibitor and moderate-intensity P-glycoprotein inhibitor, caused a 1.5-fold increase in mean AUC and 1.4-fold increase in Cmax of rivaroxaban. This increase in AUC and increase in Cmax is within normal limits and is considered clinically insignificant.
Eritromycin (500 mg 3 times/day), which moderately inhibits the CYP3A4 isoenzyme and P-glycoprotein, caused a 1.3-fold increase in the mean equilibrium AUC and Cmax of rivaroxaban. This increase in AUC and increase in Cmax varies within the normal range and is considered clinically significant.
The concomitant administration of rivaroxaban and rifampicin, a potent inducer of CYP3A4 and P-glycoprotein, resulted in approximately 50% reduction in the mean AUC of rivaroxaban and a parallel reduction in its pharmacodynamic effects. Co-administration of rivaroxaban with other potent CYP3A4 inducers (e.g., phenytoin, carbamazepine, phenobarbital, or St. John’s wort preparations) may also result in decreased plasma concentrations of rivaroxaban. Decreased plasma concentrations of rivaroxaban are considered clinically insignificant.
Additive effect was observed after combined use of enoxaparin (single dose of 40 mg) and rivaroxaban (single dose of 10 mg) with respect to the activity of antifactor Xa, which is not accompanied by additional effects with respect to blood clotting parameters (prothrombin time, ACTV). Enoxaparin did not alter the pharmacokinetics of rivaroxaban.
There was no pharmacokinetic interaction between rivaroxaban and clopidogrel (shock dose of 300 mg followed by a maintenance dose of 75 mg), but there was a clinically significant increase in bleeding time in a subgroup of patients that did not correlate with platelet aggregation or P-selectin or GPIIb/IIIa-receptor levels.
There was no clinically relevant prolongation of bleeding time after concomitant administration of rivaroxaban and 500 mg naproxen. However, more pronounced pharmacodynamic response is possible in individuals.
Food Interaction: rivaroxaban in dose of 10 mg can be taken with meals or separately.
Effect on laboratory tests: effect on coagulation parameters (prothrombin time, ACTV, Heptest) is as expected considering the mechanism of action of rivaroxaban.
Special Instructions
Special Instructions
Antithrombotic drugs, including rivaroxaban, should be used with caution in the treatment of patients at increased risk of bleeding.
In patients with severe renal impairment (CK < 30 ml/min), plasma concentrations of rivaroxaban may be significantly elevated, which may lead to an increased risk of bleeding. Because of the underlying disease, these patients are at increased risk of both bleeding and thrombosis.
The use of rivaroxaban has not been studied in clinical trials for surgical interventions undertaken for hip fractures.
If there is an unexplained decrease in hemoglobin or BP, look for the source of bleeding.
There has been no observed prolongation of the QT interval during treatment with rivaroxaban.
When performing spinal tap and epidural/spinal anesthesia for patients receiving platelet aggregation inhibitors to prevent thromboembolic complications, there is a risk of epidural or spinal hematoma, which may lead to prolonged paralysis.
The risk of these events is further increased by the use of permanent catheters or concomitant use of medications that affect hemostasis. Trauma from an epidural or spinal tap or repeated puncture may also contribute to the risk. Patients should be monitored for signs or symptoms of neurologic abnormalities (e.g., numbness or weakness in the legs, bowel or bladder dysfunction). Urgent diagnosis and treatment is needed if neurological disorders are detected.
The physician should weigh the potential benefit against risk before performing a spinal catheter intervention in patients receiving anticoagulants or preparing to receive anticoagulants for thrombosis prevention. The epidural catheter should not be removed earlier than 18 h after the last dose of rivaroxaban. Rivaroxaban should not be administered earlier than 6 h after removal of the epidural catheter. In case of traumatic puncture the administration of rivaroxaban should be postponed for 24 h.
If an invasive procedure or surgery is necessary, Xarelto should be discontinued at least 24 h before the intervention and on the basis of a physician’s report.
If the procedure cannot be postponed, the increased risk of bleeding should be evaluated against the need for an emergency intervention.
The administration of Xarelto should be resumed after an invasive procedure or surgery, provided appropriate clinical signs and adequate hemostasis are present.
The use in liver disorders
Rivaroxaban is contraindicated in patients with liver disease accompanied by coagulopathy, which causes a clinically significant risk of bleeding. Patients with other liver diseases do not require dose changes. The limited clinical data available in patients with moderate hepatic impairment (class B according to Child-Pugh classification) indicate a significant increase in pharmacological activity of the drug. No clinical data are available for patients with severe hepatic impairment (Child-Pugh class C).
The use in renal dysfunction
There is no need to reduce the dose when prescribing rivaroxaban in patients with mild (IQ 80-50 ml/min) or moderate (IQ < 50-30 ml/min) renal impairment.
Limited clinical data available in patients with severe renal impairment (KC < 30-15 ml/min) show a significant increase in rivaroxaban concentrations in these patients. Rivaroxaban should be used with caution to treat this category of patients.
The use of rivaroxaban is not recommended in patients with CK < 15 ml/min.
Safety data from preclinical studies
With the exception of effects associated with enhanced pharmacologic action (bleeding), no specific hazards to humans were found in the analysis of preclinical data from pharmacologic safety studies.
Influence on driving and operating machinery
There have been no studies of the effect of rivaroxaban on the ability to drive or operate potentially dangerous moving machinery.
In the postoperative period, fainting and dizziness have been infrequently reported. Patients who experience these adverse reactions should not drive motor vehicles or operate moving machinery.
Contraindications
Contraindications
With caution:
Side effects
Side effects
Given the pharmacological mechanism of action, use of rivaroxaban may be accompanied by an increased risk of occult or overt bleeding from any organ or tissue, which may lead to posthemorrhagic anemia. Signs, symptoms, and severity (including the possibility of death) will vary depending on the location and severity or duration of the bleeding. The risk of bleeding may increase in certain groups of patients, such as patients with uncontrolled severe AH and/or in patients taking medications that affect hemostasis.
Hemorrhagic complications may manifest as weakness, asthenia, pallor, dizziness, headache or edema of unclear etiology. Therefore, when evaluating a patient receiving anticoagulants, the likelihood of bleeding should be assessed.
Blood and lymphatic system disorders: often – anemia; sometimes – thrombocythemia.
Cardiovascular system disorders: frequent – post-procedural hemorrhage (including postoperative anemia and wound bleeding; occasionally – tachycardia, arterial hypotension (including hypotension during procedures), bleeding (including hematomas and rare cases of muscle hemorrhages), gastrointestinal hemorrhages (including hematemesis, bleeding gums, rectal bleeding, hematuria, bloody discharge from the genital tract, nasal bleeding).
The digestive system: frequently – nausea; rarely – constipation, diarrhea, abdominal pain, abdominal discomfort, dyspeptic complaints, dry mouth, vomiting; rarely – liver function disorders.
Others: sometimes localized or peripheral edema, fatigue, weakness, asthenia, fever.
Allergic reactions: sometimes – urticaria (including cases of generalized urticaria); rarely – allergic dermatitis.
CNS disorders: sometimes – dizziness, headache, syncopal conditions.
Muscular system disorders: sometimes – pain in the extremities.
Dermatological reactions: sometimes – itching (including cases of generalized itching), skin rashes.
Urinary system disorders: sometimes – renal failure (increased blood levels of creatinine, urea).
Laboratory tests: often – increase of LDH level, elevated AAT level; sometimes – increase of lipase, amylase, blood bilirubin levels, alkaline phosphate level; rarely – increase of conjugated bilirubin level (with or without concomitant increase of liver transaminases).
Overdose
Overdose
Symptoms: Rare cases of overdose have been reported when taking rivaroxaban up to 600 mg without the development of bleeding or other adverse reactions. Due to limited absorption, saturation effects are expected without further increase in mean plasma levels of rivaroxaban at hypertherapeutic doses of 50 mg or higher.
Treatment: The specific antidote for rivaroxaban is unknown. In case of overdose, activated charcoal may be used to reduce absorption of rivaroxaban. Given the intense binding to plasma proteins, rivaroxaban is not expected to be excreted by dialysis.
Treatment of bleeding: If bleeding occurs, the following steps may be taken to correct it: later administration of the next dose of rivaroxaban or withdrawal of treatment, as appropriate. T 1/2 of rivaroxaban leaves approximately 5-13 h. Appropriate symptomatic treatment should be considered, such as mechanical compression (e.g., in case of severe nasal bleeding), surgical intervention, fluid volume replenishment and hemodynamic support, blood transfusion or blood components. If these measures do not stop the bleeding, one of the following procoagulants may be prescribed:
However, to date, there is no experience with these products in the treatment of patients receiving rivaroxaban.
Protamine sulfate and vitamin K are not expected to affect the anticoagulation activity of rivaroxaban. There is no scientific rationale or experience with the use of systemic hemostatic agents (e.g., desmopresin, aprotinin, tranexamic acid, aminocaproic acid ) to reverse rivaroxaban overdose.
Pregnancy use
Pregnancy use
It is contraindicated in children under 18 years of age, pregnant and during lactation.
Pregnancy
There are no data on the use of rivaroxaban in pregnant women.
The data obtained in experimental animals showed marked maternal toxicity of rivaroxaban associated with the pharmacological action of the drug (e.g., complications in the form of hemorrhage) and leading to reproductive toxicity.
Because of the possible risk of bleeding and the ability to penetrate the placenta, rivaroxaban is contraindicated in pregnancy.
Women with preserved fertility should use effective contraception during treatment with rivaroxaban.
Lactation
There are no data on the use of rivaroxaban for the treatment of women during lactation.
The data obtained in experimental animals show that rivaroxaban is excreted with breast milk. Rivaroxaban can be used only after withdrawal of breastfeeding.
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | Bayer AG, Germany |
| Medication form | pills |
| Brand | Bayer AG |
Other forms…
Related products
Buy Xarelto, 10 mg 100 pcs with delivery to USA, UK, Europe and over 120 other countries.