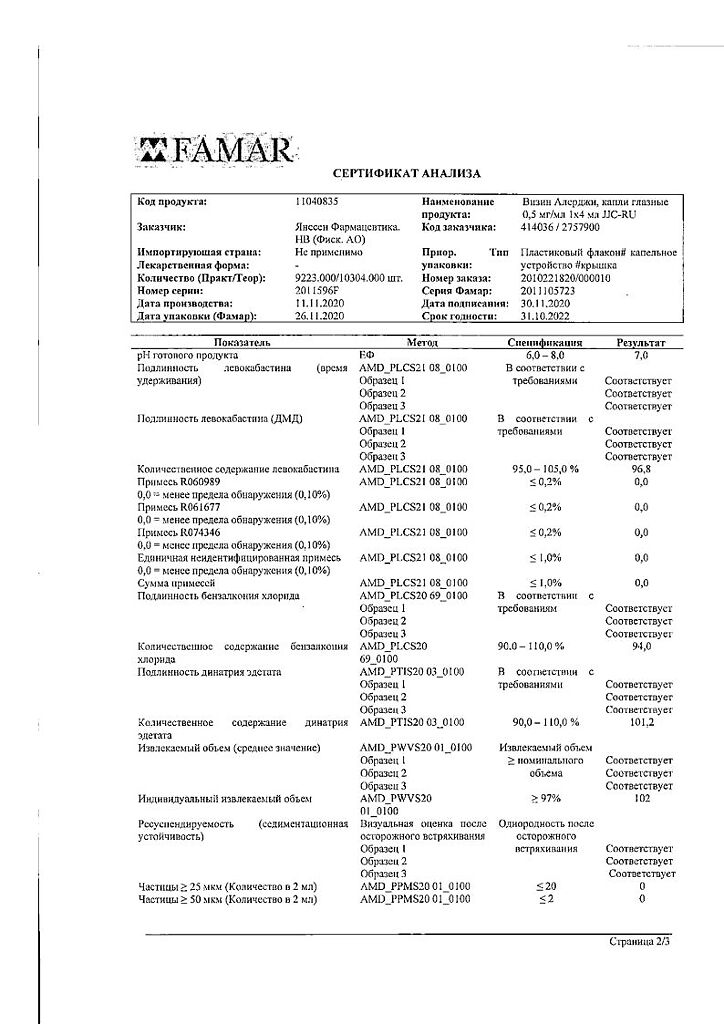
No products in the cart.
Visin Alergy, eye drops 0.05% 4 ml
€15.71 €13.09
Description
Increased tear production, Allergies, ConjunctivitisAllergic conjunctivitis.
Indications
Indications
Allergic conjunctivitis.
Special instructions
Special instructions
During the treatment period, it is not recommended to use soft (hydrophilic) contact lenses – interaction with benzalkonium chloride, which is part of the drug, is possible.
To prevent microbial contamination, avoid touching the eyelids with the pipette and close the bottle tightly.
If the medicine has become unusable or the expiration date has expired, do not throw it away! Place the medication in a bag and place it in the trash. These measures will help protect the environment!
Active ingredient
Active ingredient
Levocabastine
Composition
Composition
Active ingredient:
levocabastine hydrochloride (0.54 mg/ml) in terms of levocabastine 0.5 mg/ml (0.05%);
Excipients:
propylene glycol 48.26 µl,
sodium hydrogen phosphate 8.66 mg,
sodium hydrogen phosphate monohydrate 5.38 mg,
hypromellose (2910 3 mPa.s) 2.50 mg,
polysorbate 80 1.00 mg,
benzalkonium chloride 0.15 mg (as a 50% solution 0.03 ml),
disodium edetate 0.15 mg,
water for injections up to 1.0 ml.
Pregnancy
Pregnancy
In preclinical animal studies, levocabastine, administered systemically in doses up to 2500 times (mg/kg) higher than the recommended maximum dose for topical use in humans, did not cause embryotoxic or teratogenic effects. In animals, when systemically administered levocabastine in doses more than 5000 times (in terms of mg/kg) higher than the recommended maximum dose for topical use in humans, teratogenic properties and/or an increase in embryonic death were noted.
There are no data on the use of levocabastine in pregnant women. Therefore, it is not recommended to use Visin Alergy eye drops during pregnancy, except in cases where the expected benefit to the mother outweighs the potential risk to the fetus.
The drug passes into breast milk, so breastfeeding should be stopped during treatment.
Contraindications
Contraindications
Hypersensitivity to the components of the drug, wearing contact lenses, age up to 12 years, breastfeeding.
Side Effects
Side Effects
Adverse events that were observed in patients during clinical and post-marketing studies.
Very common (>1/10), common (>1/100, 1/1000, 1/10000, <1/1000), very rare (<1/10000, including isolated reports), frequency unknown (frequency cannot be estimated from available data).Cardiac disorders: very rare: palpitations.Visual disturbances: often: pain in the eye area, blurred vision; uncommon: swelling of the eyelid; very rare: conjunctivitis, blepharitis, conjunctival edema, conjunctival hyperemia, lacrimation.General disorders and disorders at the injection site: often: burning sensation in the eyes, eye irritation; very rarely: conjunctival hyperemia, pain in the eye area, conjunctival swelling, itching in the eye area, lacrimation, blurred vision.Immune system disorders: very rare: anaphylactic shock, angioedema, hypersensitivity.Skin and subcutaneous tissue disorders: very rare: contact dermatitis, urticaria.Nervous system disorders: very rare: headache.There have been reports of severe corneal injury and extremely rare cases of corneal decalcification when using the drug in combination with ophthalmic solutions containing phosphates.
Interaction
Interaction
Not studied.
Overdose
Overdose
Symptoms: If accidentally ingested, a decrease in blood pressure, tachycardia, and severe sedation may occur.
Treatment: Forced diuresis.
Clinical pharmacology
Clinical pharmacology
Pharmacodynamics:
Levocabastine is a selective H1-histamine receptor blocker with a long-lasting effect. The local effect occurs within 5 minutes. Vizin Allergy eliminates the typical symptoms of allergic conjunctivitis (itching, redness, swelling of the conjunctiva and eyelids, lacrimation). The action lasts up to 12 hours.
Pharmacokinetics:
After instillation into the eyes at a dose of 15 mcg/drop, about 6 mcg of levocabastine is absorbed, and the maximum concentration in the blood plasma is reached after approximately 6 hours. Levocabastine is approximately 55% bound to plasma proteins.
The main metabolite of levocabastine, acyl glucuronide, is formed by glucuronidation, which is the main route of metabolite formation.
Levocabastine is excreted primarily by the kidneys unchanged (about 70% of the absorbed amount). The half-life of levocabastine is approximately 39-70 hours.
Short product description
Short product description
Helps reduce redness, itching and swelling due to allergies 1.
1-According to the instructions for use of the drug.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use the drug after the expiration date.
Visin® Alergy eye drops should be used within one month after opening.
Manufacturer
Manufacturer
Famar A.V.E., Greece
Additional information
| Shelf life | Do not use the drug after the expiration date. Eye drops Visin® Alergy should be used within one month after opening. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Johnson & Johnson, France |
| Medication form | eye drops |
| Brand | Johnson & Johnson |
Related products
Buy Visin Alergy, eye drops 0.05% 4 ml with delivery to USA, UK, Europe and over 120 other countries.