No products in the cart.
Vinpocetine, tablets 10 mg 30 pcs
€4.94 €4.39
Description
The mechanism of action of vinpocetine consists of several elements: it improves cerebral blood flow and metabolism, has a favorable effect on the rheological properties of the blood.
The neuroprotective effect is realized by reducing the adverse cytotoxic effects of excitatory amino acids. It blocks Na+- and Ca2+-channels and NMDA- and AMPA-receptors. Selectively inhibits Ca2+-calmodulin-dependent cGMP-phosphodiesterase. Increases serotonin and noradrenaline metabolism in the brain, stimulates noradrenergic neurotransmitter system and has antioxidant effect.
It improves microcirculation in the brain by inhibiting platelet aggregation, decreasing pathologically elevated blood viscosity, increasing erythrocyte deformability and inhibiting adenosine reuptake; it promotes oxygen transfer to cells by reducing the affinity of red blood cells for it.
It selectively increases cerebral blood flow by reducing cerebral vascular resistance without significant effect on systemic blood circulation parameters (blood pressure (BP), cardiac output, heart rate, total peripheral vascular resistance); it does not cause “stealing” effect.
Indications
Indications
Neurology: Symptomatic therapy for the effects of ischemic stroke, vascular vertebrobasilar insufficiency, vascular dementia, atherosclerosis of cerebral vessels, post-traumatic, hypertensive encephalopathy.
Ophthalmology: chronic vascular diseases of the retina and choroid.
Otology: perceptual hearing loss, Meniere’s disease, tinnitus.
To avoid complications, use strictly as prescribed by a physician.
Active ingredient
Active ingredient
Composition
Composition
Active ingredient:
Vinpocetine – 10.00 mg.
Auxiliary substances:
Microcrystalline cellulose – 178.00 mg;
Pregelatinized corn starch – 60.00 mg;
Colloidal silica – 0.60 mg;
Magnesium stearate – 1.40 mg.
How to take, the dosage
How to take, the dosage
Ingestion, after meals.
The course of treatment and the dose are determined by the attending physician.
The usual daily dose is 15-30 mg (5-10 mg 3 times a day).
The starting daily dose is 15 mg. The maximum daily dose
30 mg.
No dose adjustment is required for the elderly, for hepatic or renal dysfunction.
Interaction
Interaction
No interactions have been observed with beta-adrenoblockers (pindolol), clopamide, glibenclamide, digoxin, acenocoumarol and hydrochlorothiazide.
Methyldopa may increase the hyponensive effect of vinpocetine; therefore, systematic BP control is required with their concomitant use.
While there is no data to support the possibility of interactions, caution is recommended when concomitant use with central, antiarrhythmic, and anticoagulant drugs.
Special Instructions
Special Instructions
In the presence of prolonged QT interval syndrome or concomitant use of drugs that cause prolongation of the QT interval, periodic ECG control is required. In case of severe cardiac rhythm disturbances, increased intracranial pressure, antiarrhythmic drugs, prolonged QT syndrome or concomitant use of drugs causing prolongation of the QT interval, the drug should be used with caution.
Impact on ability to concentrate: During treatment, caution should be exercised while driving motor vehicle and engaging in other potentially hazardous activities requiring increased concentration and quick psychomotor reactions.
Synopsis
Synopsis
Features
Features
Intestation
After oral administration, it is rapidly absorbed from the gastrointestinal tract. Time of reaching maximum concentration (TSmax) in blood plasma is 1 hour. Absorption occurs mainly in the proximal parts of the gastrointestinal tract. During passage through the intestinal wall it is not metabolized.
Distribution
In oral administration of radioactively labeled vinpocetine to rats, the highest concentration was found in the liver and gastrointestinal tract. The maximum concentration in tissues was observed 2-4 hours after administration. The radioactivity in the brain did not exceed the values detected in the blood.
In humans the binding to plasma proteins is 66%, bioavailability when administered orally is 7%. The volume of distribution is 246.7-88.5 L, which indicates high tissue binding. Total clearance (66.7 l/h) exceeds hepatic blood flow rate (50 l/h), indicating extrahepatic metabolism.
Metabolism
The major metabolite is apovincaminate (ABA), which is
25-30% of the parent compound. The area under the curve “concentration – time” of ABA after oral administration is twice as large as that after intravenous administration of vinpocetine. Thus, vinpocetine is subject to a pronounced “primary passage” effect through the liver. Other metabolites include: hydroxyvinpocetine, hydroxy-AVA, ABA-dioxyglycinate and their conjugates (sulfates and9 or) glucuronides). No dose adjustment is required in case of liver or renal dysfunction since vinpocetine does not cumulate.
Contraindications
Contraindications
Hypersensitivity to vinpocetine or to any of the ingredients of the drug.
Pregnancy, lactation.
Children under 18 years of age (due to insufficient data).
Side effects
Side effects
The following classification is used to determine the frequency of side effects of the drug:
Very common (⥠1/10)
Frequent (⥠1/100 and < 1/10)
Infrequent (⥠1/1000 and < 1/100)
Rarely (⥠1/10,000 and < 1/1000)
Very rarely (⥠1/10,000).
Heart: rare – myocardial ischemia/infarction, bradycardia, angina pectoris, tachycardia, extrasystole, palpitations; very rare – arrhythmia, atrial fibrillation.
Vascular disorders: infrequent – decrease of BP; rare – increase of BP, sensation of hot flashes, thrombophlebitis; very rare – BP instability.
The central nervous system: infrequent – headache; rare – dysgeusia, stupor, hemiparesis, somnolence, amnesia; very rare – tremor, spasms.
As for the visual organ: rare – edema of the optic disc; very rare – conjunctival hyperemia.
Hearing and balance: infrequent – vertigo; rare – hyperacusis, hypoacusis, tinnitus.
The digestive system: infrequent – abdominal discomfort, dry mouth, nausea; rare – dyspepsia, vomiting, constipation, diarrhea, epigastric pain; very rare – stomatitis, dysphagia.
Mental disorders: rare – sleep disorders (insomnia, increased sleepiness), anxiety; very rare – euphoria, depression.
The blood and lymphatic system: rarely – leukopenia, thrombocytopenia; very rarely – anemia, erythrocyte agglutination.
Immunological disorders: very rare – hypersensitivity.
Metabolism and nutrition disorders: infrequent – hypercholesterolemia; rarely – decreased appetite, anorexia, diabetes.
Skin and subcutaneous tissue: rarely – skin hyperemia, hyperhidrosis, pruritus, urticaria, skin rash; very rarely – dermatitis.
General disorders and disorders at the place of administration: rarely – asthenia, malaise, burning sensation; very rarely – discomfort in the chest, hypothermia.
Laboratory disorders: infrequent – decrease of BP; rare – increase of BP hypertriglyceridemia, ECG changes (ST depression, prolongation of QT interval), eosinopenia, violation of liver function tests; very rare – leukopenia, leukocytopenia, erythropenia, decreased thrombin time, weight gain.
Overdose
Overdose
There have been no recorded cases of overdose. According to the literature, the use of 60 mg of vinpocetine per day is safe. Single oral administration of 360 mg vinpocetine does not cause clinically significant cardiovascular and other reactions.
The treatment is symptomatic.
Similarities
Similarities
Additional information
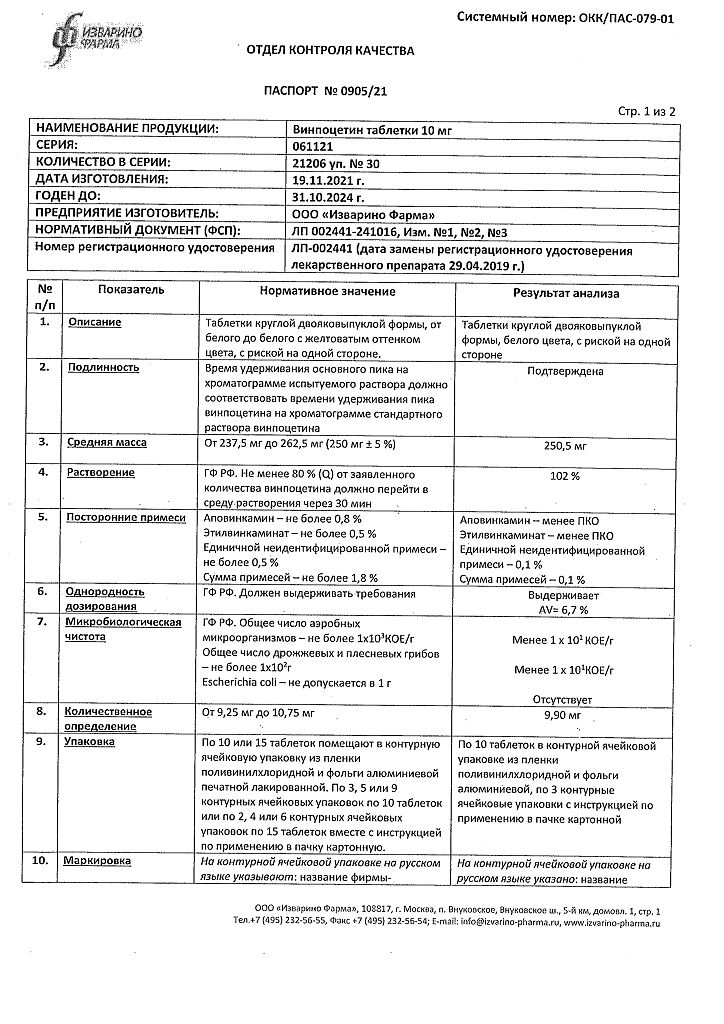
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In the dark place at a temperature not exceeding 25 °С. Store out of the reach of children. |
| Manufacturer | Izvarino Pharma, Russia |
| Medication form | pills |
| Brand | Izvarino Pharma |
Other forms…
Related products
Buy Vinpocetine, tablets 10 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.