No products in the cart.
Description
Interferon alpha-2b human recombinant has antiviral, immunomodulatory, antiproliferative properties, suppresses replication of RNA- and DNA-containing viruses. The immunomodulatory properties of interferon alfa-2b, such as increasing the phagocytic activity of macrophages and increasing the specific cytotoxicity of lymphocytes toward the target cells, determine its mediated antibacterial activity.
In the presence of ascorbic acid and alpha-tocopherol acetate the specific antiviral activity of interferon alfa-2b increases and its immunomodulatory activity increases which allows to increase the effectiveness of the body’s own immune response to pathogenic microorganisms.
In application of the preparation the level of secretory immunoglobulins of class A increases, the level of immunoglobulin E normalizes and the endogenous system of interferon alfa-2b is restored. Ascorbic acid and alpha-tocopherol acetate, being highly active antioxidants, possess anti-inflammatory, membrane stabilizing and regenerating properties.
With VIFERON® administration it was found out that no side effects appeared during parenteral administration of interferon-alpha-2b preparations, no antibodies neutralizing anti-viral activity of interferon-alpha-2b were formed. VIFERON® usage in combination therapy enables to decrease therapeutic doses of antibacterial and hormonal drugs, as well as decrease the toxic effects of this therapy.
Cocoa butter contains phospholipids which do not allow the use of synthetic toxic emulsifiers and the presence of polyunsaturated fatty acids facilitates the introduction and dissolution of the product.
Indications
Indications
acute respiratory viral infections, including influenza, including those complicated by bacterial infection, in children and adults as part of complex therapy;
infectious and inflammatory diseases of newborns, including premature babies: pneumonia (bacterial, viral, chlamydial), meningitis (bacterial, viral), sepsis, intrauterine infection (chlamydia, herpes, cytomegalovirus infection, enterovirus infection, candidiasis, including visceral, mycoplasmosis) as part of complex therapy;
chronic viral hepatitis B, C, D in children and adults as part of complex therapy, including in combination with the use of plasmapheresis and hemosorption for chronic viral hepatitis of pronounced activity, complicated by cirrhosis of the liver;
infectious and inflammatory diseases of the urogenital tract (chlamydia, cytomegalovirus infection, ureaplasmosis, trichomoniasis, gardnerellosis, papillomavirus infection, bacterial vaginosis, recurrent vaginal candidiasis, mycoplasmosis) in adults, including pregnant women, as part of complex therapy;
primary or recurrent herpetic infection of the skin and mucous membranes, localized form, mild and moderate course, including the urogenital form, in adults, including pregnant women;
viral meningitis in children over 4 years of age as part of complex therapy;
chronic bacterial prostatitis in adults as part of complex therapy.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Cytokine.
ATX code L03АВ05
Pharmacological action
Human recombinant interferon alpha-2b has antiviral, immunomodulatory, antiproliferative properties, suppresses the replication of RNA and DNA viruses. The immunomodulatory properties of interferon alpha-2b, such as increased phagocytic activity of macrophages and increased specific cytotoxicity of lymphocytes to target cells, determine its indirect antibacterial activity.
In the presence of ascorbic acid and alpha-tocopherol acetate, the specific antiviral activity of interferon increases
alpha-2b, its immunomodulatory effect is enhanced, which makes it possible to increase the effectiveness of the body’s own immune response to pathogenic microorganisms. When using the drug, the level of secretory immunoglobulins of class A increases, the level of immunoglobulin E normalizes, and the functioning of the endogenous interferon alpha-2b system is restored. Ascorbic acid and alpha-tocopherol acetate, being highly active antioxidants, have anti-inflammatory, membrane stabilizing, and regenerating properties. It has been established that when using the drug VIFERON® there are no side effects that occur with parenteral administration of interferon alpha-2b preparations, no antibodies are formed that neutralize the antiviral activity of interferon
alpha-2b. The use of the drug VIFERON® as part of complex therapy makes it possible to reduce therapeutic doses of antibacterial and hormonal drugs, as well as reduce the toxic effects of this therapy.
Cocoa butter contains phospholipids, which make it possible not to use synthetic toxic emulsifiers in production, and the presence of polyunsaturated fatty acids facilitates the administration and dissolution of the drug.
Special instructions
Special instructions
The category of children with an increased risk of complications and a long course of acute respiratory viral infection, including influenza, includes: children who are often and long-term ill with a frequency of episodes more than 4 times a year and lasting more than 5 days, children with chronic diseases in the acute stage or in the stage of unstable remission with frequent exacerbations.
Impact on the ability to drive vehicles and engage in other activities
Not installed.
Active ingredient
Active ingredient
Interferon alpha-2b
Composition
Composition
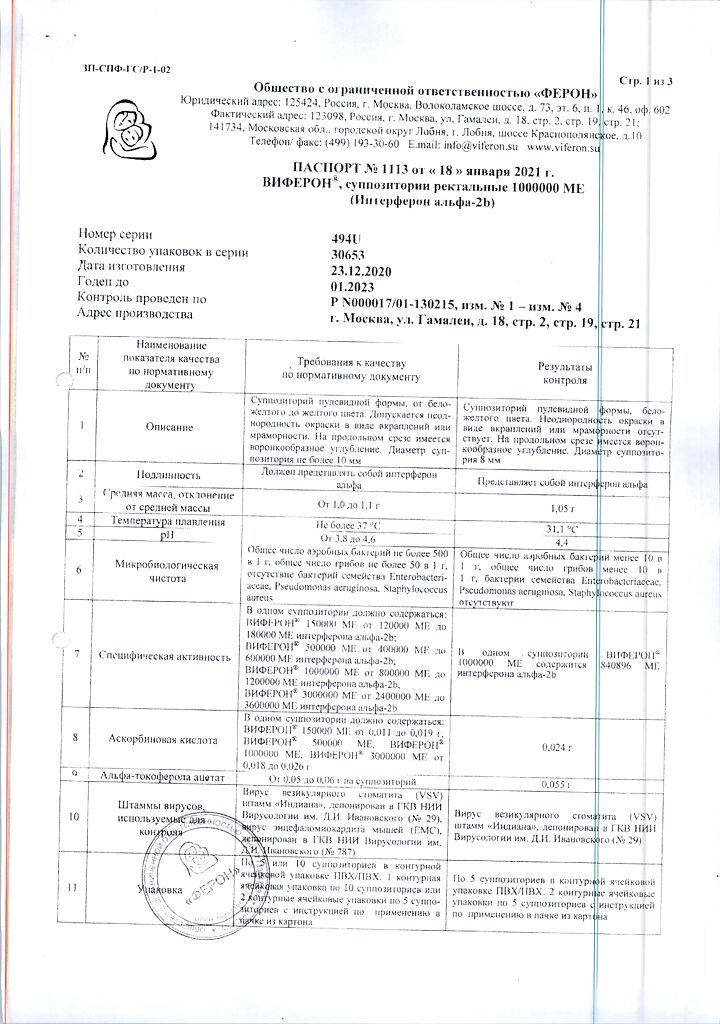
1 suppository VIFERON® 150000 IU contains the active substance: human recombinant interferon alpha-2b 150000 IU, excipients: ascorbic acid 0.0054 g, sodium ascorbate 0.0108 g, alpha-tocopherol acetate 0.055 g, disodium edetate dihydrate 0.0001 g, polysorbate-80 0.0001 g, cocoa butter 0.1958 g, confectionery fat or cocoa butter substitute up to 1 g.
1 suppository VIFERON® 500000 IU contains the active substance: human recombinant interferon alpha-2b 500000 IU, excipients: ascorbic acid 0.0081 g, sodium ascorbate 0.0162 g, alpha-tocopherol acetate 0.055 g, disodium edetate dihydrate 0.0001 g, polysorbate-80 0.0001 g, cocoa butter 0.1941 g, confectionery fat or cocoa butter substitute up to 1 g.
1 suppository VIFERON® 1000000 IU contains the active substance: human recombinant interferon alpha-2b 1000000 IU, excipients: ascorbic acid 0.0081 g, sodium ascorbate 0.0162 g, alpha-tocopherol acetate 0.055 g, disodium edetate dihydrate 0.0001 g, polysorbate-80 0.0001 g, cocoa butter 0.1941 g, confectionery fat or cocoa butter substitute up to 1 g.
1 suppository VIFERON® 3000000 IU contains the active substance: human recombinant interferon alpha-2b 3000000 IU, excipients: ascorbic acid 0.0081 g, sodium ascorbate 0.0162 g, alpha-tocopherol acetate 0.055 g, disodium edetate dihydrate 0.0001 g, polysorbate-80 0.0001 g, cocoa butter 0.1941 g, confectionery fat or cocoa butter substitute up to 1 g.
Pregnancy
Pregnancy
The drug is approved for use from the 14th week of pregnancy. There are no restrictions for use during breastfeeding.
Contraindications
Contraindications
Hypersensitivity to any of the components of the drug.
Side Effects
Side Effects
In rare cases, allergic reactions (skin rashes, itching) may develop. These phenomena are reversible and disappear 72 hours after stopping the drug.
Interaction
Interaction
VIFERON®, rectal suppositories, is compatible and goes well with all medications used in the treatment of the above diseases (antibiotics, chemotherapy drugs, glucocorticosteroids).
Overdose
Overdose
Not installed.
Storage conditions
Storage conditions
At temperatures from 2 to 8 ºС.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use the drug after the expiration date.
Manufacturer
Manufacturer
Feron, Russia
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | The drug should be stored in a dry place protected from light at 2° to 8°C. |
| Manufacturer | Feron, Russia |
| Medication form | rectal suppositories |
| Brand | Feron |
Other forms…
Related products
Buy Viferon, rectal 1000000 me 10 pcs with delivery to USA, UK, Europe and over 120 other countries.