No products in the cart.
Viagra, 50 mg 4 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Pharmacokinetics
The pharmacokinetics of sildenafil are dose-dependent when taken orally in the recommended dose range. Absorption
After oral administration, sildenafil is rapidly absorbed from the gastrointestinal tract. Absolute bioavailability of sildenafil is on average about 41% (25-63%). Maximum plasma concentration of Stach when taken orally on an empty stomach is reached within 30-120 minutes (on average 60 minutes). When taken in combination with fatty foods the absorption rate is reduced; Cmax is reduced by 29% on average, and the time to reach maximum concentration (Ttah) is prolonged by 60 min.
Distribution
The volume of distribution of sildenafil in the equilibrium state averages 105 liters. Binding to plasma proteins of sildenafil and its main circulating N-demethyl metabolite is approximately 96% and is independent of the total drug concentration. Ninety minutes after sildenafil administration, less than 0.0002% of the dose (188 ng on average) is detected in semen. Metabolism
Sildenafil is metabolized mainly in the liver under the action of microsomal cytochromeP450 isoenzymes: CYP3A4 (main pathway) and CYP2C9. The main circulating active metabolite formed as a result of N-demethylation of sildenafil undergoes further metabolism. The plasma concentration of the metabolite is about 40% of the sildenafil concentration. Excretion
The total clearance of sildenafil from the body is 41 l/hour, and the final elimination half-life is 3-5 hours. After oral administration sildenafil is excreted as metabolites mainly in the feces (about 80% of the dose) and to a lesser extent in the urine (about 13% of the dose). Elderly patients
In elderly patients (65 years and older) sildenafil clearance is decreased and free sildenafil plasma concentrations are about 40% higher than in younger patients (18-45 years). Renal failure In mild (creatinine clearance 50-80 ml/min) and moderate (creatinine clearance 30-49 ml/min) degree of renal failure ^ sildenafil pharmacokinetics after a single oral administration of 50 mg does not change. In severe renal insufficiency (creatinine clearance < 30 ml/min) sildenafil clearance is decreased, resulting in approximately twofold increase in the area under the pharmacokinetic curve (AUC) and Stah (88%).
Hepatic impairment In patients with cirrhosis (Child-Pugh A and B), sildenafil clearance is decreased, resulting in an 84% increase in AUC and 47% increase in Stach.
Pharmacodynamics
. The active ingredient of Viagra – sildenafil is a potent selective inhibitor of cycloguanosine monophosphate (cGMP) – specific phosphodiesterase type 5 (PDE5). Sildenafil has no direct relaxant effect on the isolated cavernous body. Sildenafil enhances the relaxing effect of nitric oxide (NO) by inhibiting FDEF5, which is responsible for the breakdown of cGMP in the cavernous body.
The result is relaxation of the smooth muscles and increased blood flow in the corpora cavernosa.
Indications
Indications
Treatment of erectile dysfunction characterized by the inability to achieve or maintain an erection of the penis sufficient for satisfactory intercourse.
The drug Viagra is effective only in the presence of sexual stimulation.
Active ingredient
Active ingredient
Composition
Composition
Active substance:
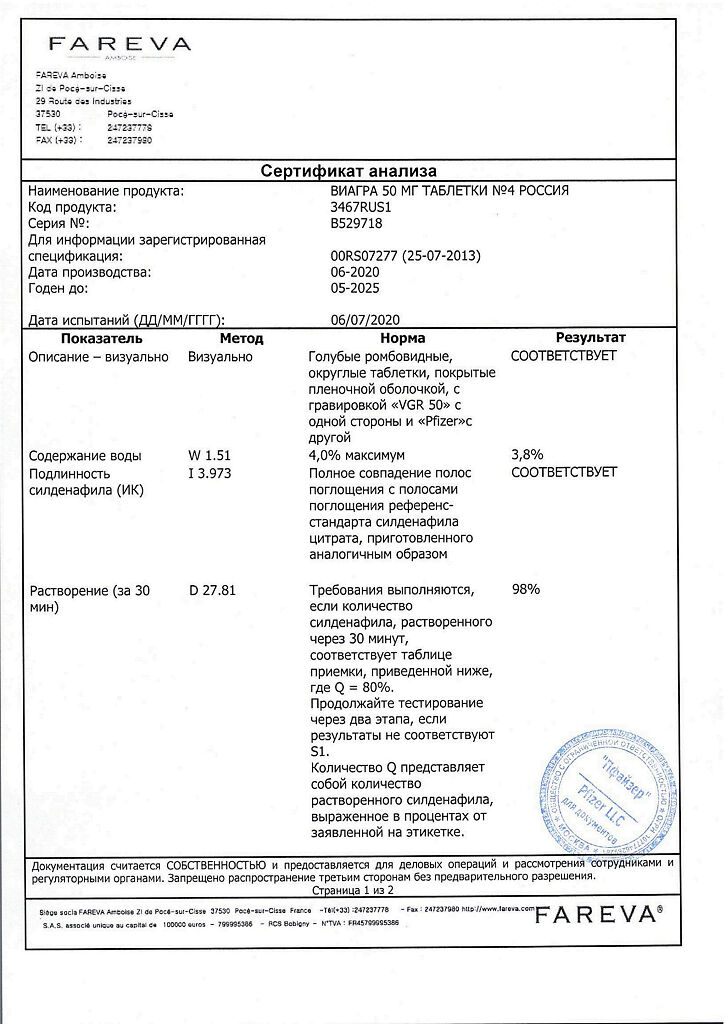
sildenafil (in the form of sildenafil citrate) 50 mg;
excipients:
Microcrystalline cellulose,
calcium hydrophosphate (anhydrous),
croscarmellose sodium,
magnesium stearate,
Opadry blue and Opadry clear film coatings.
How to take, the dosage
How to take, the dosage
The drug is taken orally.
The recommended dose for most patients is 50 mg; the drug is taken as needed about 1 hour before sexual activity. Depending on efficacy and tolerability, the dose may be increased to 100 mg or decreased to 25 mg.
The maximum recommended dose is 100 mg.
The maximum recommended frequency of use is once daily.
Special Instructions
Special Instructions
To diagnose erectile dysfunction, determine its possible causes, and choose an appropriate treatment, a complete medical history and a thorough physical examination must be taken.
The treatment of erectile dysfunction should be used with caution in patients with anatomic penile deformities (angulation, cavernous fibrosis, Peyronie’s disease), or in patients with risk factors for priapism (sickle cell anemia, multiple myeloma, leukemia).
The drugs intended to treat erectile dysfunction should not be prescribed for men for whom sexual activity is undesirable.
Contraindications
Contraindications
– Hypersensitivity to any component of the drug.
– Use in patients receiving nitric oxide donators, organic nitrates or nitrites in any form continuously or intermittently.
– Age under 18 years.
– Not intended for use in women.
With caution: anatomical deformity of the penis (including. angulation, cavernous fibrosis or Peyronne’s disease), diseases predisposing to the development of priapism (such as sickle cell anemia, multiple myeloma. leukemia, thrombocythemia), bleeding disorders, exacerbation of peptic ulcer disease, hereditary retinitis pigmentosa, heart failure, unstable angina pectoris, myocardial infarction, stroke, or life-threatening arrhythmias in the last 6 months, hypertension (BP > 170/100 mm Hg) or hypotension (BP < 90/50 mm Hg).
Side effects
Side effects
Adverse events are usually transient and mild to moderately severe. The frequency of adverse events increases with increasing dose.
In the body in general: asthenia, pain, abdominal pain, back pain, infection, flu-like syndrome.
Cardiovascular system: vasodilation (side effect reported in clinical trials).
Digestive system disorders: diarrhea, nausea.
Muscular system disorders: joint pain, muscle pain.
CNS and peripheral nervous system disorders: dizziness (side effect reported in clinical trials), increased muscle tone, insomnia.
Respiratory system disorders: nasal congestion, pharyngitis, rhinitis (side effect reported in clinical studies), sinusitis, respiratory tract infections, respiratory dysfunction.
Dermatological reactions: rash.
Sensory organs: changes in vision: mild and transient, mainly changes in the color of objects, as well as increased perception of light and blurred vision (side effect reported in clinical studies), conjunctivitis.
Urinary system disorders: urinary tract infections.
Perior genital system disorders: disorders of prostate function.
When using the drug in doses higher than recommended, the side effects were similar to those noted above, but were usually more common.
The following are the adverse events reported during post-marketing use.
Cardiovascular disorders: arterial hypotension, syncope, tachycardia, palpitations.
Digestive system disorders: vomiting (side effect reported in clinical trials).
Anxiety of the sexual system: prolonged erection and/or priapism.
Sensory system disorders: pain in the eyes, redness of the eyes.
Others: allergic reactions.
Pregnancy use
Pregnancy use
Similarities
Similarities
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | In a dry place, at a temperature no higher than 30 °C |
| Manufacturer | Farève Amboise, France |
| Medication form | pills |
| Brand | Farève Amboise |
Other forms…
Related products
Buy Viagra, 50 mg 4 pcs with delivery to USA, UK, Europe and over 120 other countries.