No products in the cart.
Venarus 500,500 mg 60 pcs
€39.43 €32.85
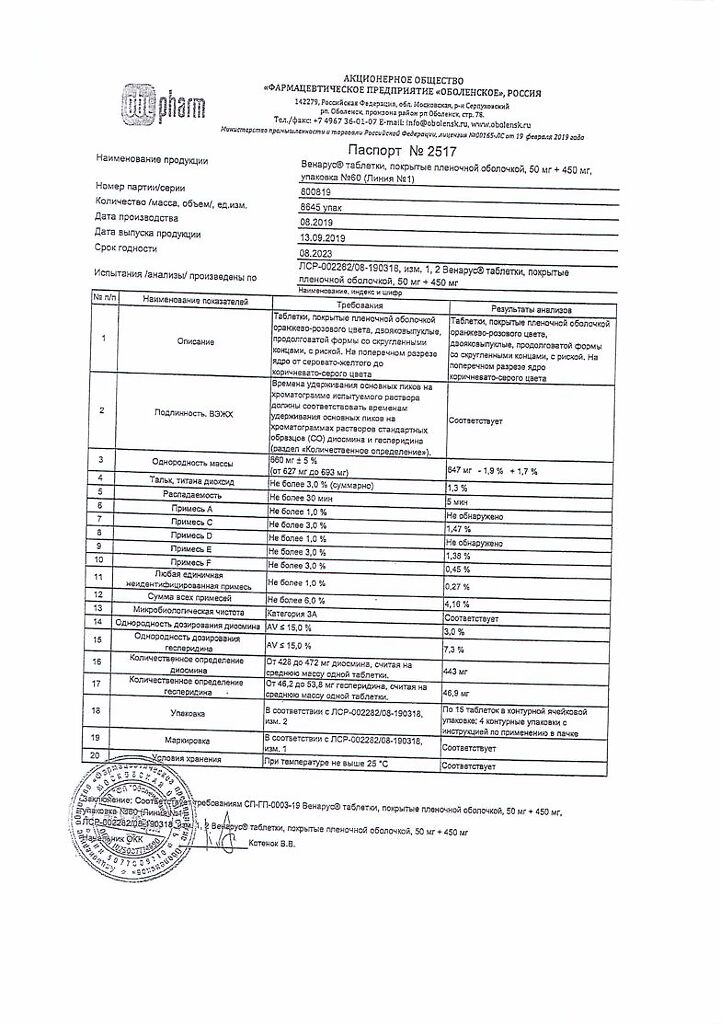
Description
It has angioprotective and venotonic effect.
Reduces veins, increases their tone and reduces venous stasis; reduces permeability, capillary fragility and increases their resistance; improves microcirculation and lymphatic flow.
When used systematically it reduces clinical manifestations of chronic venous insufficiency of the lower limbs of organic and functional nature.
.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
1 tablet contains:
Active substances:
diosmin – 0.45 g,
hesperidin – 0.05 g.
Auxiliary substances (core):
gelatin, magnesium stearate,
microcrystalline cellulose,
starch, sodium glycolate,
talc.
Auxiliary substances (shell):
polyethylene glycol 6000,
magnesium stearate,
hydroxypropyl methylcellulose,
sodium lauryl sulfate,
titanium dioxide,
iron oxide red,
iron oxide yellow.
How to take, the dosage
How to take, the dosage
60 pcs. 50 mg+450 mg.
Interaction
Interaction
It has not been noted.
The attending physician should be informed of all medications taken.
Special Instructions
Special Instructions
In case of exacerbation of hemorrhoids the prescription of this drug does not replace the specific treatment of other anal disorders. The duration of treatment should not exceed the periods specified in the section “Dosage and administration”. If symptoms do not disappear after the recommended course of therapy, proctologic examination should be performed and the therapy used should be revised.
In the presence of venous circulatory disorders, the maximum effect of treatment is achieved by combining therapy with a healthy (balanced) lifestyle: it is advisable to avoid long periods of sun exposure, prolonged exposure to the sun, as well as reducing excessive body weight. Walking and, in some cases, wearing special stockings helps to improve blood circulation.
Influence on the ability to drive vehicles, and other mechanisms requiring increased concentration
No effect.
Features
Features
The main excretion of the drug occurs in the feces. About 14% of the taken drug amount is excreted with urine on average.
The elimination half-life is 11 hours.
The drug undergoes active metabolism, which is confirmed by the presence of phenolic acids in the urine.
Contraindications
Contraindications
Side effects
Side effects
The incidence of adverse reactions is as follows:
Unwanted reactions whose incidence cannot be estimated from available data are labeled “frequency unknown”.
Central nervous system: rare – dizziness, headache, general malaise; frequency unknown – seizures.
Gastrointestinal tract: frequent – diarrhea, dyspepsia, nausea, vomiting; infrequent – colitis; frequency unknown – abdominal pain.
Respiratory system, chest and mediastinum: frequency unknown – sore throat, chest pain.
Skin disorders: rare – rash, itching, urticaria; frequency unknown – allergic dermatitis, hyperemia, isolated swelling of the face, lips, eyelids, in exceptional cases angioedema.
If any of the adverse reactions mentioned in the instructions worsen, or if you notice other adverse reactions not mentioned in the instructions, inform your physician.
Overdose
Overdose
No cases of overdose have been described.
In case of overdose, seek medical attention immediately.
Pregnancy use
Pregnancy use
Pregnancy
There have been no animal experiments showing teratogenic effects. To date, there have been no reports of any side effects when using the drug in pregnant women.
Breast-feeding
Because of the lack of data concerning excretion of the drug into the breast milk, it is not recommended for breast-feeding women to take the drug.
Similarities
Similarities
Additional information
| Weight | 0.073 kg |
|---|---|
| Shelf life | 2 years |
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Alium JSC, Russia |
| Medication form | pills |
| Brand | Alium JSC |
Other forms…
Related products
Buy Venarus 500,500 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.