No products in the cart.
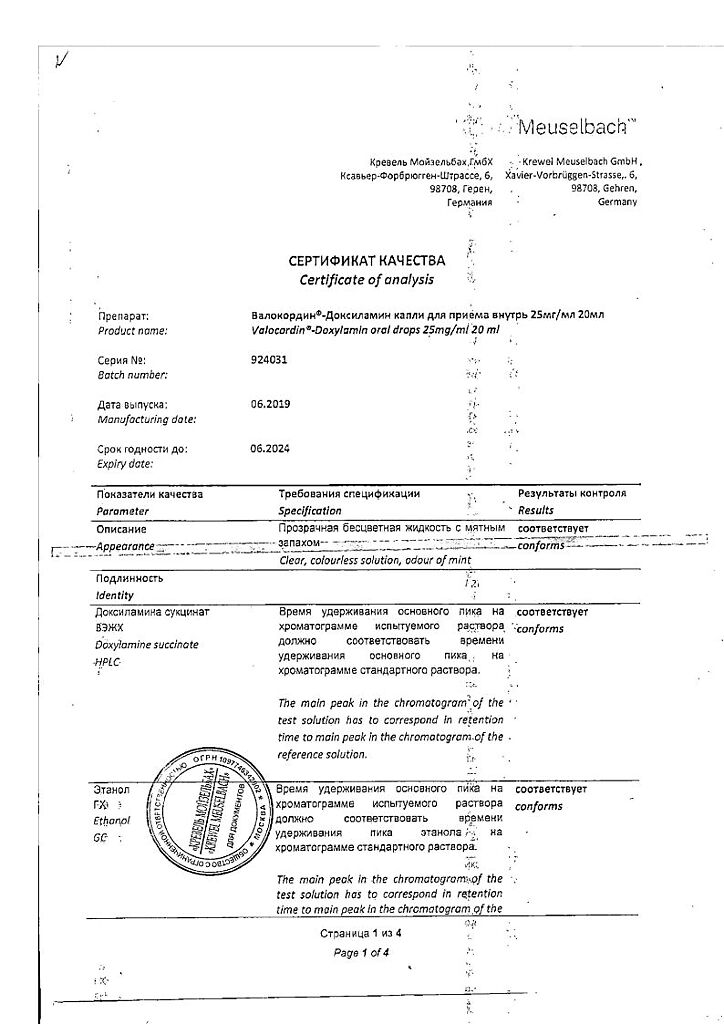
Valokordin-Doxylamine, drops 25 mg/ml 20 ml
€10.41 €8.67
Out of stock
(E-mail when Stock is available)
EAN: 4030031898341
SKU: 228028
Categories: Medicine, Neurology and Psychiatry, Sedatives and hypnotics
Description
Pharmacodynamics
A blocker of H1-histamine receptors from the group of ethanolamines. The drug has soporific, sedative and m-cholinoblocking effects. It shortens the time of falling asleep, increases the duration and quality of sleep, but does not change the phase of sleep.
Doxylamine is quickly and almost completely absorbed immediately after oral administration. Action begins within 30 minutes, the maximum serum concentration of 99 ng/ml is detected 2.0-2.4 hours after an oral dose of 25 mg. The duration of action is from 3 to 6 hours.
Pharmacokinetics
Mainly metabolized in the liver. It penetrates well through histohematic barriers (including the blood-brain barrier). The elimination half-life varies from 10.1 to 12 hours. The main part of the dose (about 60%) is excreted unchanged in the urine, partially in the intestine.
Indications
Indications
Symptomatic treatment of periodic sleep disorders (difficulty falling asleep and waking up at night) in adults.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
H1-histamine receptor blocker from the ethanolamine group. The drug has a hypnotic, sedative and m-anticholinergic effect. Reduces the time to fall asleep, increases the duration and quality of sleep, without changing the sleep phases.
Doxylamine is rapidly and almost completely absorbed immediately after oral administration. The action begins within 30 minutes, the maximum serum concentration of 99 ng/ml is detected 2.0-2.4 hours after oral administration of a 25 mg dose. Duration of action is from 3 to 6 hours.
Pharmacokinetics
Mainly metabolized in the liver. Penetrates well through histohematic barriers (including the blood-brain barrier). The half-life ranges from 10.1 to 12 hours. The main part of the dose (approx. 60%) is excreted unchanged in the urine, and partially in the intestines.
Special instructions
Special instructions
Valocordin-Doxylamine contains 55% ethanol (alcohol) by volume, i.e. up to 900 mg per dose, which corresponds to up to 21.87 ml of beer or 9.11 ml of wine per dose. It is dangerous for people with liver diseases, alcoholism, epilepsy, as well as for pregnant women and children.
Impact on the ability to drive vehicles and operate machinery
Due to possible drowsiness during the daytime, you should avoid driving vehicles, operating machinery and other activities that require quick mental and motor reactions.
Active ingredient
Active ingredient
Doxylamine
Composition
Composition
1 ml of the drug (22 drops) contains:
Active substance:
doxylamine succinate – 25.0 mg.
Excipients:
ethanol 96% – 450.0 mg,
peppermint oil – 1.4 mg,
purified water – 449.7 mg.
Pregnancy
Pregnancy
Valocordin-Doxylamine can be used during pregnancy only when the expected benefit to the mother outweighs the possible risk to the fetus.
Breastfeeding should be discontinued during treatment as the active substance is excreted into breast milk.
Contraindications
Contraindications
Hypersensitivity to doxylamine and other components of the drug.
Angle-closure glaucoma.
Prostatic hyperplasia, urinary retention.
Childhood and adolescence.
With caution: pregnancy, liver disease, alcoholism, traumatic brain injury or brain disease.
Side Effects
Side Effects
The frequency of side effects is given according to the following scale:
Very frequent – 1/10 prescriptions (> 10%).
Frequent – 1/100 prescriptions (> 1% and < 10%).
Infrequent – 1/1000 prescriptions (> 0.1% and < 1%).
Rare - 1/10,000 prescriptions (> 0.01% and < 0.1%).
Very rare - less than 1/10,000 prescriptions (<0.01%).
The most commonly reported side effects: constipation, dry mouth, accommodation disturbances, urinary retention, daytime drowsiness (in this case, the dose of the drug should be reduced).
Less likely (from 0.01% to 10%) are likely:
Cardiovascular system: tachycardia, cardiac arrhythmias, decreased or increased blood pressure, and decompensation of an existing cardiac arrhythmia may occur. Changes on the ECG are possible.
Hematopoietic system and lymphatic system: in extremely rare cases, after the use of antihistamines, changes in the blood picture in the form of leukopenia, thrombocytopenia and hemolytic anemia may appear; aplastic anemia and agranulocytosis have been very rarely observed.
Nervous system: dizziness, headache.
Visual disturbances: increased intraocular pressure.
Disorders of the ear and ear labyrinth: tinnitus.
Respiratory system: thickening of bronchial secretions, bronchial obstruction and bronchospasm which can lead to deterioration of respiratory function.
Gastrointestinal tract: nausea, vomiting, diarrhea, loss or increase of appetite, epigastric pain. In very rare cases, life-threatening paralytic ileus may occur. Liver dysfunction (cholestatic jaundice) has been reported during therapy with histamine H1 receptor blockers.
Urinary system: urinary disturbance.
Skin: allergic skin reactions, disorders of photosensitivity and thermoregulation may be observed.
Endocrine system: In patients with pheochromocytoma, taking antihistamines may lead to an increase in the release of catecholamines.
General disorders: feeling of nasal congestion, fatigue, muscle weakness.
Mental disorders: Undesirable effects, which depend on individual sensitivity and the dose taken, include: delayed reaction, impaired concentration, low mood. In addition, there is a possibility of the appearance of so-called “paradoxical” reactions, such as anxiety, nervous agitation, psycho-emotional stress, insomnia, night “nightmares”, confusion, hallucinations, tremors. After prolonged daily use, sleep disturbances may worsen due to sudden discontinuation of therapy.
Development of tolerance: Loss of effectiveness (tolerance) may occur after long-term use of sedatives.
Dependence: like taking other sedatives, taking Valocordin-Doxylamine can lead to the development of physical and mental dependence. The risk of dependence increases with the dose and duration of treatment and increases in patients with a history of alcohol, drug or drug dependence. Even after completing short-term treatment with Valocordin-Doxylamine, temporary sleep disturbances may occur again due to sudden withdrawal. Therefore, it is recommended, if necessary, to complete treatment by gradually reducing the dose.
Anterograde amnesia: Even in therapeutic doses, sedatives can provoke anterograde amnesia, especially in the first hours after administration. The risk increases with increasing dose, but can be reduced by sufficiently long uninterrupted sleep (7-8 hours).
Interaction
Interaction
When taking the drug Valocordin-Doxylamine simultaneously with antidepressants, barbiturates, benzodiazepines, clonidine, narcotic analgesics (analgesics, antitussives), neuroleptics, anxiolytics, sedatives, H1-histamine receptor blockers, central antihypertensive drugs, thalidomide, baclofen, pizotifen the inhibitory effect on the central nervous system increases.
When taken simultaneously with m-anticholinergic drugs (atropine, tricyclic antidepressants, antiparkinsonian drugs, atropine antispasmodics, disopyramide, phenothiazine antipsychotics), the risk of side effects such as urinary retention, constipation, and dry mouth increases.
Since alcohol enhances the sedative effect of most H1-histamine receptor blockers, incl. and the drug Valocordin-Doxylamine, it is necessary to avoid the simultaneous consumption of alcoholic beverages and medications containing alcohol.
Overdose
Overdose
Symptoms: daytime drowsiness, dilated pupils (mydriasis), accommodation disturbances, dry mouth, redness of the skin of the face and neck (hyperemia), increased body temperature (hyperthermia), sinus tachycardia, disorder of consciousness, hallucinations, decreased mood, anxiety, impaired coordination of movements, trembling (tremor), involuntary movements (athetosis), convulsions (epileptic syndrome), coma
Involuntary movements are sometimes a precursor to seizures, which may indicate severe poisoning. Even in the absence of seizures, severe doxylamine poisoning can cause rhabdomyolysis, which is often accompanied by severe renal failure. In such cases, standard therapy with constant monitoring of creatine phosphokinase activity is indicated. If symptoms of poisoning appear, consult a doctor immediately.
Treatment: symptomatic (m-cholinomimetics, etc.), activated carbon (in the amount of 50 g) is indicated as a first aid remedy.
Storage conditions
Storage conditions
3 years. Shelf life after opening the bottle: 6 months.
Shelf life
Shelf life
Store at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Manufacturer
Manufacturer
Crevel Meuselbach GmbH, Germany
Additional information
| Shelf life | Store at the temperature not more than 25 °С. Keep out of reach of children. |
|---|---|
| Conditions of storage | 3 years. Shelf life after opening the bottle: 6 months. |
| Manufacturer | Krevel Meuselbach GmbH, Germany |
| Medication form | oral drops |
| Brand | Krevel Meuselbach GmbH |
Related products
Buy Valokordin-Doxylamine, drops 25 mg/ml 20 ml with delivery to USA, UK, Europe and over 120 other countries.