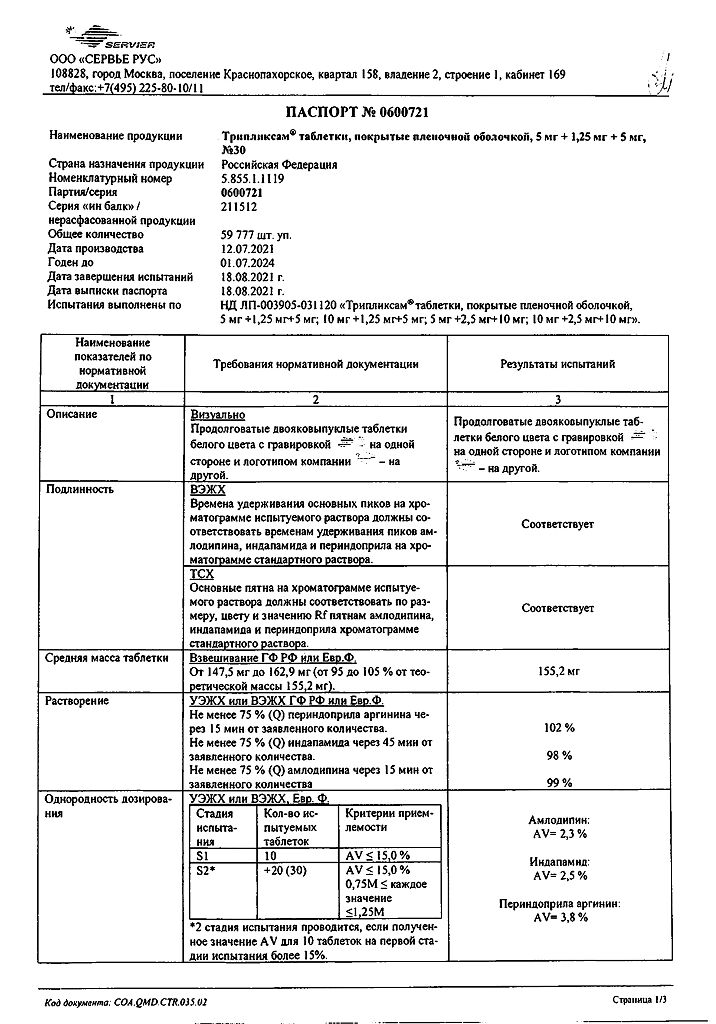
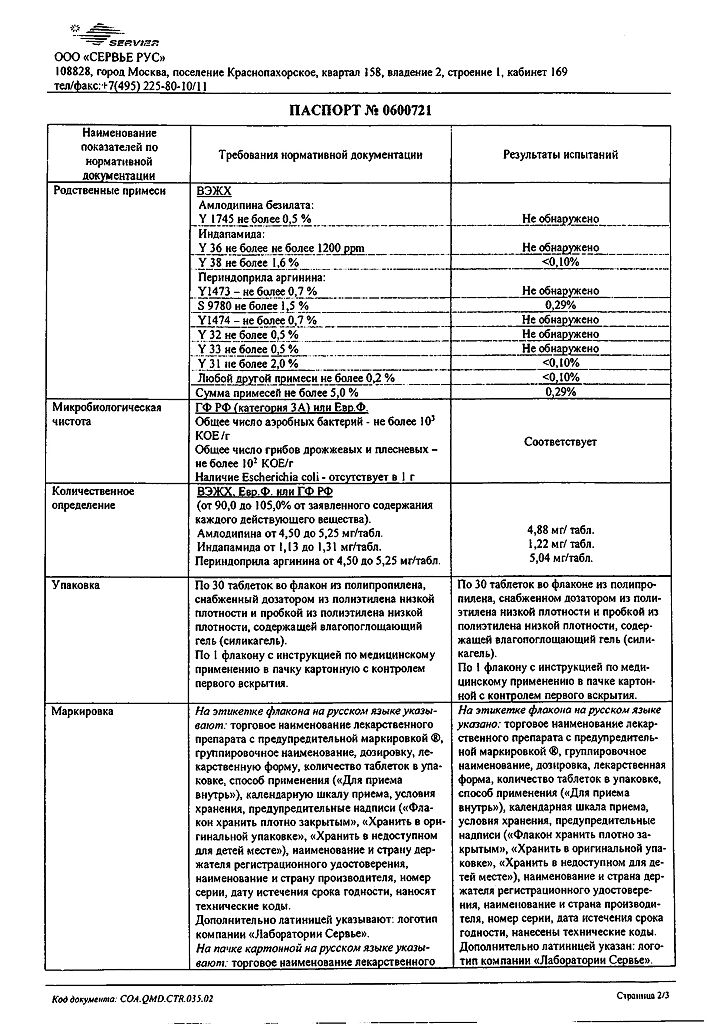
No products in the cart.
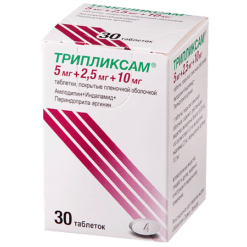
Triplixam, 5 mg+1.25 mg+5 mg 30 pcs.
€23.14 €19.29
Description
Triplixam® is a combination drug that includes three antihypertensive components, each of which complements the action of the others in controlling blood pressure in patients with arterial hypertension. Amlodipine is a “slow” calcium channel blocker (CMCB), a dihydropyridine derivative, indapamide is a sulfonamide diuretic, perindopril arginine is an inhibitor of the enzyme that converts angiotensin I into angiotensin II (ACE inhibitor)
Indications
Indications
As therapy in patients with arterial hypertension when blood pressure is reduced while taking amlodipine, indapamide and perindopril in the same doses.
Pharmacological effect
Pharmacological effect
Triplixam® is a combination drug that includes three antihypertensive components, each of which complements the action of the others in controlling blood pressure in patients with arterial hypertension. Amlodipine – a blocker of “slow” calcium channels (SCBC), a dihydropyridine derivative, indapamide – a sulfonamide diuretic, perindopril arginine – an inhibitor of the enzyme that converts angiotensin I into angiotensin II (ACE inhibitor)
Special instructions
Special instructions
All precautions associated with taking individual components of the drug should be taken into account when using their fixed combination as part of the drug Triplixam®.
Amlodipine
Chronic heart failure
Patients with chronic heart failure should be treated with caution.
When using amlodipine in patients with chronic heart failure III and functional class according to the NYHA classification, pulmonary edema may develop. Slow calcium channel blockers, including amlodipine, should be used with caution in patients with chronic heart failure due to a possible increased risk of cardiovascular adverse events and mortality.
In patients with severe chronic heart failure (NYHA functional class IV), treatment should begin with lower doses and under close medical supervision.
Patients with arterial hypertension and coronary heart disease should not stop taking beta-blockers: an ACE inhibitor should be used in conjunction with beta-blockers.
Hypertensive crisis
The effectiveness and safety of amlodipine in hypertensive crisis has not been established.
Indapamide
Hepatic encephalopathy
In the presence of liver dysfunction, taking thiazide and thiazide-like diuretics can lead to the development of hepatic encephalopathy. In this case, you should immediately stop taking the diuretic.
Photosensitivity
Cases of photosensitivity reactions have been reported while taking thiazide and thiazide-like diuretics (see section “Side effects”). If a photosensitivity reaction develops while taking the drug, treatment should be discontinued. If it is necessary to continue diuretic therapy, it is recommended to protect the skin from exposure to sunlight or artificial ultraviolet rays.
Content of calcium ions in blood plasma
Thiazide and thiazide-like diuretics can reduce the excretion of calcium ions by the kidneys and lead to a slight and temporary increase in the content of calcium ions in the blood plasma. Severe hypercalcemia may be a consequence of previously undiagnosed hyperparathyroidism. In such cases, you should stop taking diuretics and conduct a study of the function of the parathyroid glands (see section “Side effects”).
Uric acid
In patients with increased concentrations of uric acid in the blood plasma during therapy, the frequency of gout attacks may increase.
Perindopril
Potassium-sparing diuretics, potassium supplements, potassium-containing table salt substitutes and food supplements
The simultaneous administration of perindopril and potassium-sparing diuretics, as well as potassium preparations, potassium-containing table salt substitutes and food additives is not recommended (see section “Interaction with other drugs”).
Dual blockade of the renin-angiotensin-aldosterone system (RAAS)
There is evidence of an increased risk of arterial hypotension, hyperkalemia and renal dysfunction (including acute renal failure) when ACE inhibitors are used simultaneously with ARB II or aliskiren. Therefore, double blockade of the RAAS as a result of combining an ACE inhibitor with ARA II or aliskiren is not recommended (see sections “Interaction with other drugs” and “Pharmacodynamics”). If a double blockade is necessary, it should be performed under the strict supervision of a specialist with regular monitoring of renal function, plasma electrolyte levels and blood pressure. ACE inhibitors should not be used concomitantly with ARB II in patients with diabetic nephropathy.
Neutropenia/agranulocytosis/thrombocytopenia/anemia
There are reports of the development of neutropenia/agranulocytosis, thrombocytopenia and anemia while taking ACE inhibitors. In patients with normal renal function and in the absence of other aggravating factors, neutropenia rarely develops. Perindopril should be used with extreme caution in patients with systemic connective tissue diseases, while taking immunosuppressants, allopurinol or procainamide, or their combination, especially in patients with impaired renal function.
Some of these patients developed severe infections, in some cases resistant to intensive antibiotic therapy. When prescribing perindopril to such patients, periodic monitoring of white blood cell counts is recommended, and patients should report any signs of infectious diseases (eg, sore throat, fever) to the doctor (see section “Side effects”).
Hypersensitivity/angioedema
When taking ACE inhibitors, including perindopril, in rare cases, the development of angioedema of the face, extremities, lips, tongue, glottis and/or larynx may occur. This can happen at any time during therapy. If symptoms appear, the drug should be stopped immediately and the patient should be observed until signs of edema completely disappear. If the swelling affects only the face and lips, it usually resolves on its own, although antihistamines may be used to treat symptoms.
Angioedema, accompanied by swelling of the larynx, can be fatal. Swelling of the tongue, glottis or larynx can lead to airway obstruction, in which case intensive care should be given immediately. If such symptoms appear, you should immediately administer a solution of epinephrine (adrenaline) 1:1000 (0.3–0.5 ml) and/or ensure airway patency. The patient should be under medical supervision until symptoms disappear completely and permanently.
Black patients had a higher incidence of angioedema while taking ACE inhibitors compared to other races.
In patients with a history of angioedema not associated with taking ACE inhibitors. the risk of its development may be increased when taking the drug (see section “Contraindications”). There are reports of rare cases of the development of angioedema of the intestine during therapy with ACE inhibitors. In this case, patients experienced abdominal pain as an isolated symptom or in combination with nausea and vomiting, in some cases without previous angioedema of the face and with normal levels of C1-esterase. The diagnosis was made using abdominal computed tomography, ultrasound, or at the time of surgery. Symptoms resolved after discontinuation of ACE inhibitors. Therefore, in patients with abdominal pain receiving ACE inhibitors, when carrying out differential diagnosis, it is necessary to take into account the possibility of developing angioedema of the intestine.
Anaphylactoid reactions during desensitization
There are isolated reports of the development of prolonged, life-threatening anaphylactoid reactions in patients receiving ACE inhibitors during desensitizing therapy with hymenoptera venom (bees, wasps). ACE inhibitors should be used with caution in patients with a history of allergies or a tendency to allergic reactions undergoing desensitization procedures, and the use of an ACE inhibitor should be avoided in patients receiving immunotherapy with hymenoptera venom. However, an anaphylactoid reaction can be avoided by temporarily discontinuing the ACE inhibitor at least 24 hours before the start of the desensitization procedure.
Anaphylactoid reactions during LDL apheresis
In rare cases, life-threatening anaphylactoid reactions may occur in patients receiving ACE inhibitors during LDL apheresis using dextran sulfate. To prevent an anaphylactoid reaction, ACE inhibitor therapy should be temporarily discontinued before each apheresis procedure.
Hemodialysis
Anaphylactoid reactions have been reported in patients receiving ACE inhibitors during hemodialysis using high-flux membranes (eg, AN69®). Therefore, it is advisable to use a different type of membrane or use an antihypertensive agent of a different pharmacotherapeutic group.
Pregnancy
Taking ACE inhibitors during pregnancy is contraindicated. If continued therapy with ACE inhibitors is necessary, patients should switch to other types of antihypertensive therapy with an established safety profile when taken during pregnancy. If pregnancy occurs, ACE inhibitors should be stopped immediately and, if necessary, alternative antihypertensive therapy should be started (see sections “Contraindications” and “Use during pregnancy and lactation”).
Cough
During therapy with an ACE inhibitor, a dry cough may occur. The cough persists for a long time while taking drugs of this group and disappears after their discontinuation. If a patient develops a dry cough, one should be aware of the possible iatrogenic nature of this symptom. If the physician believes that ACE inhibitor therapy is necessary for the patient, continuing the drug may be considered.
Mitral stenosis/aortic stenosis/hypertrophic obstructive cardiomyopathy
ACE inhibitors should be administered with caution to patients with left ventricular outflow tract obstruction.
Ethnic differences
Perindopril, like other ACE inhibitors, apparently has a less pronounced hypotensive effect in patients of the Negroid race compared to representatives of other races. Perhaps this difference is due to the fact that black patients with arterial hypertension are more likely to have low renin activity.
Surgery/General anesthesia
The use of ACE inhibitors in patients undergoing surgery under general anesthesia can lead to a significant decrease in blood pressure, especially when using general anesthetic agents that have an antihypertensive effect.
It is recommended, if possible, to stop taking long-acting ACE inhibitors, including perindopril, one day before surgery.
Patients with renovascular hypertension
The treatment method for renovascular hypertension is revascularization. However, the use of ACE inhibitors has a beneficial effect in patients both awaiting surgery and in cases where surgery is not possible.
When using the drug Triplixam® in patients with existing or suspected renal artery stenosis, treatment should be started in a hospital setting with low doses with constant monitoring of the condition of the kidneys and potassium levels in the blood, since such patients may develop functional renal failure, which disappears when therapy is stopped.
Atherosclerosis
The risk of arterial hypotension exists in all patients, however, special care should be taken when using the drug in patients with coronary heart disease and cerebrovascular insufficiency. In such patients, treatment should begin with low doses of the drug.
Perindopril/indapamide.
Lithium preparations
The simultaneous use of a combination of perindopril and indapamide with lithium preparations is not recommended (see section “Interaction with other drugs”).
Arterial hypotension and water-electrolyte imbalance
The presence of initial hyponatremia is associated with the risk of sudden development of arterial hypotension (especially in patients with renal artery stenosis). Therefore, when monitoring patients, attention should be paid to possible symptoms of dehydration and decreased plasma electrolytes, for example, after diarrhea or vomiting. Such patients require regular monitoring of blood plasma electrolyte levels. In case of severe arterial hypotension, intravenous administration of 0.9% sodium chloride solution may be required.
Transient arterial hypotension is not a contraindication for continued therapy. After restoration of blood volume and blood pressure, you can resume therapy using low doses of the combination, or use the components of the drug in monotherapy.
All diuretics can cause hyponatremia. which sometimes leads to serious complications. Hyponatremia at the initial stage may not be accompanied by clinical symptoms, so regular laboratory monitoring is necessary. More frequent monitoring of sodium ion levels is indicated in elderly patients and patients with liver cirrhosis (see sections “Side effects” and “Overdose”).
Patients with diabetes
In patients with type 1 diabetes mellitus (risk of spontaneous increases in potassium ions), treatment should begin with lower doses and under close medical supervision.
When prescribing the drug to patients with diabetes mellitus receiving oral hypoglycemic agents or insulin, regular monitoring of plasma glucose concentrations is necessary during the first month of therapy. It is necessary to monitor blood glucose levels in patients with diabetes mellitus, especially in the presence of hypokalemia.
Amlodipine/perindopril
Liver failure
In rare cases, cholestatic jaundice occurs while taking ACE inhibitors. As this syndrome progresses, fulminant liver necrosis develops, sometimes with death. The mechanism of development of this syndrome is unclear. If jaundice or a significant increase in the activity of liver enzymes occurs in patients taking ACE inhibitors, you should stop taking the ACE inhibitor and consult a doctor (see section “Side Effects”).
In patients with impaired liver function, T½ and AUC of amlodipine are increased. Taking amlodipine should be started with the lowest doses and precautions should be taken both at the beginning of treatment and when increasing the dose. In patients with severe hepatic impairment, the dose should be increased gradually, ensuring careful monitoring of the clinical condition.
Triplixam® has not been studied in patients with liver failure. Considering the effect of each component included in the drug separately, Triplixam® is contraindicated in patients with severe liver failure, and also requires special caution when prescribed to patients with moderate and mild liver failure.
Amlodipine/indapamide/perindopril
Renal dysfunction
The drug is contraindicated in patients with severe renal failure (creatinine clearance less than 30 ml/min) (see section “Contraindications”).
In patients with moderate renal failure (creatinine clearance 30–60 ml/min), the use of Triplixam® in dosages containing 10 mg of perindopril and 2.5 mg of indapamide (i.e., Triplixam® dosages of 5 mg + 2.5 mg + 10 mg and 10 mg + 2.5 mg + 10 mg) is contraindicated.
Some patients with arterial hypertension without previous obvious renal impairment may develop laboratory signs of functional renal failure during therapy. In this case, treatment with the drug should be stopped with the further possibility of resuming combination therapy using low doses of the drug, or using the components of the drug in monotherapy. Such patients require regular monitoring of the content of potassium ions and creatinine in the blood serum – 2 weeks after the start of therapy and then every 2 months. Renal failure occurs more often in patients with severe chronic heart failure or underlying renal impairment, including renal artery stenosis.
Triplixam® is not recommended for patients with bilateral renal artery stenosis or arterial stenosis of a single functioning kidney.
There is a risk of arterial hypotension and/or renal failure (in the presence of chronic heart failure, dehydration and a decrease in the content of electrolytes in the blood plasma, etc.): in some pathological conditions, significant activation of the RAAS may be observed, especially with severe hypovolemia and a decrease in the content of electrolytes in the blood plasma (against the background of a salt-free diet or long-term use of diuretics), in patients with initially low blood pressure, renal artery stenosis (including bilateral), chronic heart failure or cirrhosis of the liver with edema and ascites.
Blockade of the RAAS by ACE inhibitors may be accompanied by a sharp decrease in blood pressure and/or an increase in the concentration of creatinine in the blood plasma, indicating the development of functional renal failure. These phenomena are more often observed when taking the first dose of the drug or during the first two weeks of therapy. Sometimes these conditions develop acutely and the time of their onset may vary. In such cases, it is recommended to resume therapy starting with lower doses, gradually increasing them. In patients with coronary artery disease and cerebrovascular diseases, a sharp decrease in blood pressure can lead to myocardial infarction or cerebrovascular accident.
Thiazide and thiazide-like diuretics are fully effective only in patients with normal or slightly impaired renal function (plasma creatinine concentration in adult patients below 25 mg/l or 220 µmol/l). In elderly patients, creatinine levels should be assessed taking into account age, weight and gender.
At the beginning of treatment with diuretics, patients may experience a temporary decrease in glomerular filtration rate and an increase in the concentration of urea and creatinine in the blood plasma due to hypovolemia and hyponatremia. This transient functional renal failure is not dangerous for patients with unchanged renal function, but its severity may increase in patients with underlying renal failure. Patients with renal failure can take amlodipine in standard doses. Changes in plasma concentrations of amlodipine do not correlate with the degree of renal failure.
No special studies have been conducted on the use of Triplixam® in renal failure. When using the drug Triplixam® for renal failure, the effects noted when taking individual components of the drug should be taken into account.
Content of potassium ions in blood plasma
Concomitant therapy with indapamide, perindopril and amlodipine does not prevent the development of hypokalemia, especially in patients with diabetes mellitus or renal failure. As with the use of other antihypertensive drugs in combination with a diuretic, regular monitoring of the content of potassium ions in the blood plasma is necessary.
Hyperkalemia may develop in some patients during treatment with ACE inhibitors, including perindopril. Risk factors for hyperkalemia include renal failure, impaired renal function, advanced age (>70 years), diabetes mellitus, certain concomitant conditions (dehydration, acute cardiac decompensation, metabolic acidosis), concomitant use of potassium-sparing diuretics (such as spironolactone, eplerenone, triamterene, amiloride), potassium supplements or potassium-containing substitutes table salt, as well as the use of other drugs that help increase the content of potassium ions in the blood plasma (for example, heparin). The use of potassium supplements/preparations, potassium-sparing diuretics, and potassium-containing table salt substitutes can lead to a significant increase in potassium levels in the blood, especially in patients with reduced renal function. Hyperkalemia can lead to serious, sometimes fatal, heart rhythm disturbances. If combined use of the above drugs is necessary, treatment should be carried out with caution, against the background of regular monitoring of the content of potassium ions in the blood serum (see section “Interaction with other drugs”). Therapy with thiazide and thiazide-like diuretics is associated with a risk of hypokalemia. Hypokalemia (less than 3.4 mmol/l) should be avoided in the following categories of high-risk patients: elderly and/or malnourished patients (even if they are not receiving concomitant drug therapy), patients with cirrhosis of the liver with edema and ascites, patients with coronary heart disease, chronic heart failure. Hypokalemia in these patients increases the toxic effect of cardiac glycosides and increases the risk of arrhythmia.
The risk group also includes patients with a prolonged QT interval, and it does not matter whether this increase is caused by congenital causes or the effect of drugs.
Hypokalemia, like bradycardia, contributes to the development of severe cardiac arrhythmias, in particular polymorphic ventricular tachycardia of the “pirouette” type, which can be fatal. In all the cases described above, regular monitoring of the content of potassium ions in the blood plasma is necessary. The first measurement of potassium ion content should be carried out within the first week from the start of therapy.
If hypokalemia is detected, appropriate treatment should be prescribed.
Elderly patients
Before starting to take the drug, it is necessary to assess the functional activity of the kidneys and the content of potassium ions in the blood plasma. At the beginning of therapy, the dose of the drug is selected taking into account the degree of reduction in blood pressure, especially in the case of a decrease in circulating blood volume (CBV) and loss of electrolytes. Such measures allow you to avoid a sharp decrease in blood pressure.
In elderly patients, increasing the dose should be done with caution (see sections “Dosage and Administration” and “Pharmacokinetics”).
Impact on the ability to drive vehicles and machinery
Due to the possibility of weakness and dizziness while using the drug Triplixam®, care must be taken when driving vehicles and working with other technical devices that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Amlodipine, Indapamide, Perindopril
Contraindications
Contraindications
Hypersensitivity to the active and auxiliary substances included in the drug, sulfonamide derivatives, dihydropyridine derivatives, other ACE inhibitors, any other substances included in the drug;
Patients on hemodialysis;
Untreated heart failure in the stage of decompensation;
Severe renal failure (creatinine clearance (CC) less than 30 ml/min);
Moderate renal failure (creatinine clearance (CC) less than 60 ml/min) for the dosage of the perindopril/indapamide combination 10 mg/2.5 mg (i.e. Triplixam® 5 mg + mg + 10 mg and Triplixam® 10 mg + 2.5 mg + 10 mg);
Angioedema (Quincke’s edema) with a history of taking ACE inhibitors (see section “Special instructions”);
Hereditary/idiopathic angioedema;
Pregnancy (see section “Use during pregnancy and breastfeeding”);
Breastfeeding period (see section “Use during pregnancy and breastfeeding”);
Hepatic encephalopathy:
Severe liver failure;
Hypokalemia;
Severe arterial hypotension (systolic blood pressure less than 90 mm Hg);
Shock (including cardiogenic);
Left ventricular outflow tract obstruction (eg, clinically significant aortic stenosis);
Hemodynamically unstable heart failure after acute myocardial infarction;
Concomitant use with aliskiren-containing drugs in patients with diabetes mellitus or impaired renal function (glomerular filtration rate (GFR) < 60 ml/min/1.73 m2 body surface area) (see sections “Interaction with other drugs” and “Pharmacodynamics”);
Bilateral renal artery stenosis, stenosis of the artery of a single kidney;
Concomitant use with drugs that can cause polymorphic ventricular tachycardia of the “pirouette” type;
Concomitant use with drugs that prolong the QT interval;
Concomitant use with potassium-sparing diuretics, potassium and lithium preparations, in patients with elevated potassium levels in the blood plasma;
Age up to 18 years (efficacy and safety have not been established).
Side Effects
Side Effects
The most common adverse reactions reported during treatment with perindopril, indapamide and amlodipine as monotherapy were: dizziness, headache, paresthesia, vertigo, drowsiness, visual disturbances, tinnitus, palpitations, flushing of the face, decreased blood pressure (and effects associated with hypotension), cough, shortness of breath, gastrointestinal disorders (abdominal pain, constipation, diarrhea, taste disturbance, nausea, dyspepsia, vomiting), pruritus, rash, maculopapular rash, muscle cramps, swelling in the ankle area, asthenia, edema and fatigue.
Overdose
Overdose
There is no information on overdose of Triplixam®.
Storage conditions
Storage conditions
At a temperature not higher than 25°C. Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Manufacturer
Manufacturer
Servier Rus LLC, Russia
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25°C. Keep out of reach of children. |
| Manufacturer | Servier Rus LLC, Russia |
| Medication form | pills |
| Brand | Servier Rus LLC |
Other forms…
Related products
Buy Triplixam, 5 mg+1.25 mg+5 mg 30 pcs. with delivery to USA, UK, Europe and over 120 other countries.