No products in the cart.
Trichopol, vaginal tablets 500 mg 10 pcs
€12.68 €11.10
EAN: 5903060006119
SKU: 206578
Categories: Gynecology and Obstetrics, Medicine, Trichomoniasis and malaria
Description
Antiprotozoal drug with antibacterial activity, a derivative of 5-nitroimidazole. Mechanism of action consists in biochemical reduction of 5-nitrogroup of metronidazole by intracellular transport proteins of anaerobic microorganisms and protozoa. The reduced 5-nitrogroup of metronidazole interacts with the DNA of the microbial cell, inhibiting the synthesis of their nucleic acids, which leads to the death of the microorganisms.
Metronidazole is active against Trichomonas vaginalis, Entamoeba histolytica, Gardnerella vaginalis, Giardia intestinalis, Lamblia spp.Bacteroides spp. (including Bacteroides fragilis, Bacteroides distasonis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides vulgatus), Fusobacterium spp, Veillonella spp., Prevotella spp. (Prevotella bivia, Prevotella buccae, Prevotella disiens); some Gram-positive microorganisms (Eubacterium spp., Clostridium spp., Peptococcus spp., Peptostreptococcus spp.) The MPC for these strains is 0.125-6.25 µg/ml.
In combination with amoxicillin it shows activity against Helicobacter pylori (amoxicillin suppresses the development of resistance to metronidazole).
Metronidazole has no bactericidal effect against most bacteria and facultative anaerobes, fungi and viruses. In the presence of mixed flora (aerobes and anaerobes) metronidazole shows synergism with antibiotics effective against common aerobes.
Metronidazole increases the sensitivity of tumors to radiation, causes sensitization to ethanol (disulfiram-like action), stimulates reparative processes.
Indications
Indications
For local treatment:
nonspecific vaginitis;
bacterial vaginosis;
trichomonas vaginitis.
Pharmacological effect
Pharmacological effect
Antiprotozoal drug with antibacterial activity, a derivative of 5-nitroimidazole. The mechanism of action is the biochemical reduction of the 5-nitro group of metronidazole by intracellular transport proteins of anaerobic microorganisms and protozoa. The reduced 5-nitro group of metronidazole interacts with the DNA of the cell of microorganisms, inhibiting the synthesis of their nucleic acids, which leads to the death of microorganisms.
Metronidazole is active against Trichomonas vaginalis, Entamoeba histolytica, Gardnerella vaginalis, Giardia intestinalis, Lamblia spp.; as well as obligate anaerobes Bacteroides spp. (including Bacteroides fragilis, Bacteroides distasonis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides vulgatus), Fusobacterium spp., Veillonella spp., Prevotella spp. (Prevotella bivia, Prevotella buccae, Prevotella disiens); some gram-positive microorganisms (Eubacterium spp., Clostridium spp., Peptococcus spp., Peptostreptococcus spp.). The MIC for these strains is 0.125-6.25 μg/ml.
In combination with amoxicillin, it is active against Helicobacter pylori (amoxicillin suppresses the development of resistance to metronidazole).
Metronidazole does not have a bactericidal effect against most bacteria and facultative anaerobes, fungi and viruses. In the presence of mixed flora (aerobes and anaerobes), metronidazole exhibits synergism with antibiotics that are effective against common aerobes.
Metronidazole increases the sensitivity of tumors to radiation, causes sensitization to ethanol (disulfiram-like effect), and stimulates reparative processes.
Special instructions
Special instructions
Simultaneous treatment of the sexual partner with metronidazole is recommended, regardless of whether he has manifestations of the disease.
During treatment with the drug, it is recommended to abstain from sexual intercourse.
If there are indications in the anamnesis of changes in the composition of peripheral blood, as well as when using the drug in high doses and/or during long-term treatment, monitoring of a general blood test is necessary.
Metronidazole may cause immobilization of treponemes, resulting in false-positive Nelson test results (TPI).
Impact on the ability to drive vehicles and operate machinery
The possibility of dizziness should be taken into account when prescribing the drug to patients engaged in potentially hazardous activities (especially vehicle drivers).
Active ingredient
Active ingredient
Metronidazole
Composition
Composition
1 tablet metronidazole 500 mg
Excipients:
microcrystalline cellulose,
povidone,
crospovidone,
colloidal silicon dioxide,
stearic acid.
Pregnancy
Pregnancy
Trichopolum is contraindicated for use in the first trimester of pregnancy. The use of the drug in the second and third trimesters is possible only in cases where the expected benefit to the mother outweighs the potential risk to the fetus.
If it is necessary to use Trichopolum during lactation, breastfeeding should be stopped.
Contraindications
Contraindications
blood diseases;
leukopenia (including history);
impaired coordination of movements;
organic lesions of the central nervous system (including epilepsy);
liver failure (for use in high doses);
pregnancy (first trimester);
lactation (breastfeeding);
hypersensitivity to metronidazole or other nitroimidazole derivatives.
Side Effects
Side Effects
Local reactions: itching, burning, pain and irritation in the vagina; thick, white, mucous vaginal discharge (odorless or with a slight odor), frequent urination; after discontinuation of the drug – development of vaginal candidiasis.
From the digestive system: nausea, changes in taste, metallic taste in the mouth, dry mouth, decreased appetite, abdominal cramps, nausea, vomiting, constipation or diarrhea.
From the side of the central nervous system: headache, dizziness.
From the hematopoietic system: leukopenia or leukocytosis.
Allergic reactions: urticaria, itching, rash.
Other: rarely – red-brown coloring of urine due to the presence of a water-soluble pigment formed as a result of the metabolism of metronidazole; a burning sensation or irritation of the penis in a sexual partner.
Interaction
Interaction
The drug is compatible with sulfonamides and antibiotics.
Metronidazole causes ethanol intolerance, so alcohol should be avoided during treatment.
You should not combine metronidazole with disulfiram, since the interaction of these drugs may cause depression of consciousness and the development of mental disorders.
When used simultaneously with indirect anticoagulants (including warfarin), metronidazole enhances their effect, which leads to an increase in prothrombin time.
It is not recommended to use metronidazole in combination with non-depolarizing muscle relaxants (vecuronium bromide).
Under the influence of barbiturates, the effectiveness of metronidazole may decrease due to acceleration of its metabolism in the liver.
Cimetidine inhibits the metabolism of metronidazole, which can lead to an increase in its concentration in the blood plasma and an increased risk of adverse reactions.
When taken simultaneously with lithium preparations, it is possible to increase its concentration in the blood plasma.
Overdose
Overdose
Data on overdose of the drug Trichopol® are not provided.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Polpharma JSC, Poland
Additional information
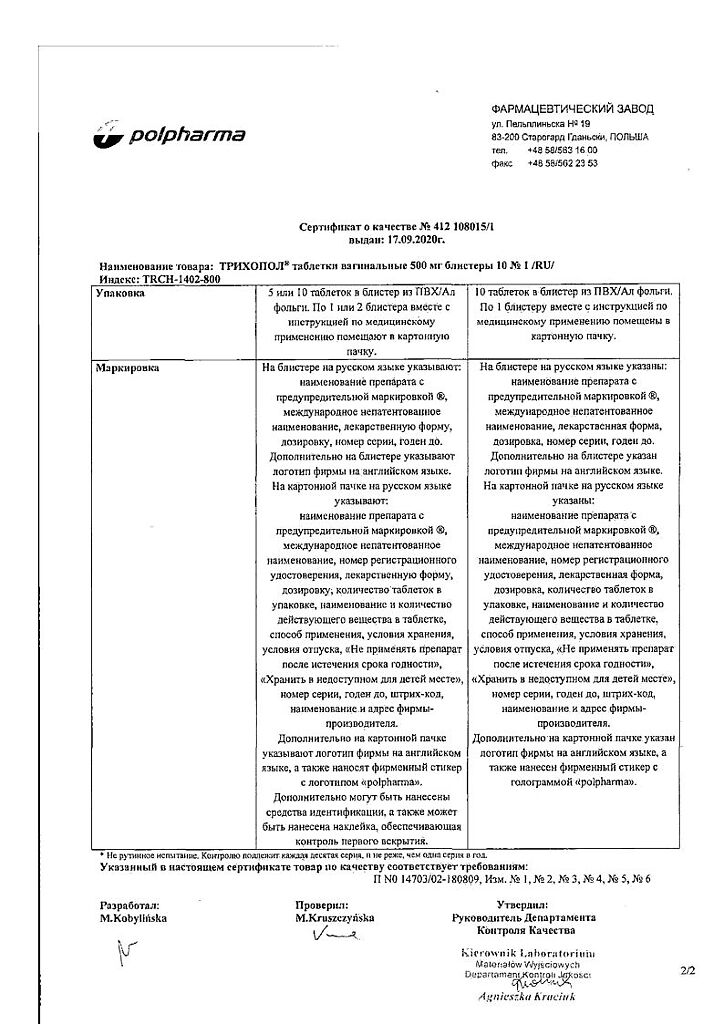
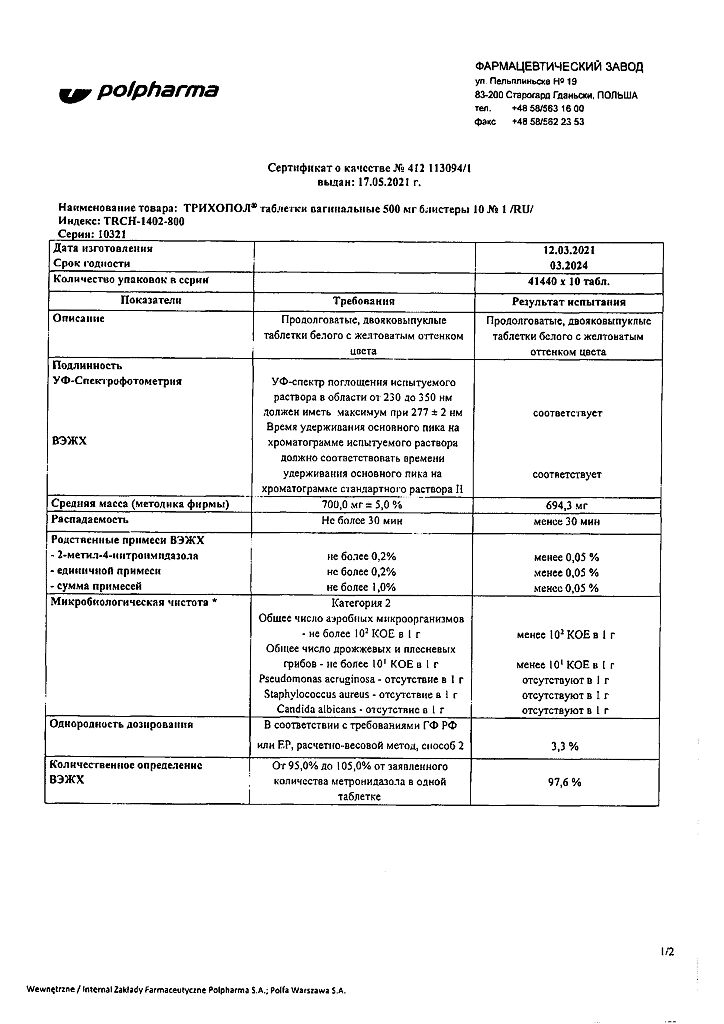
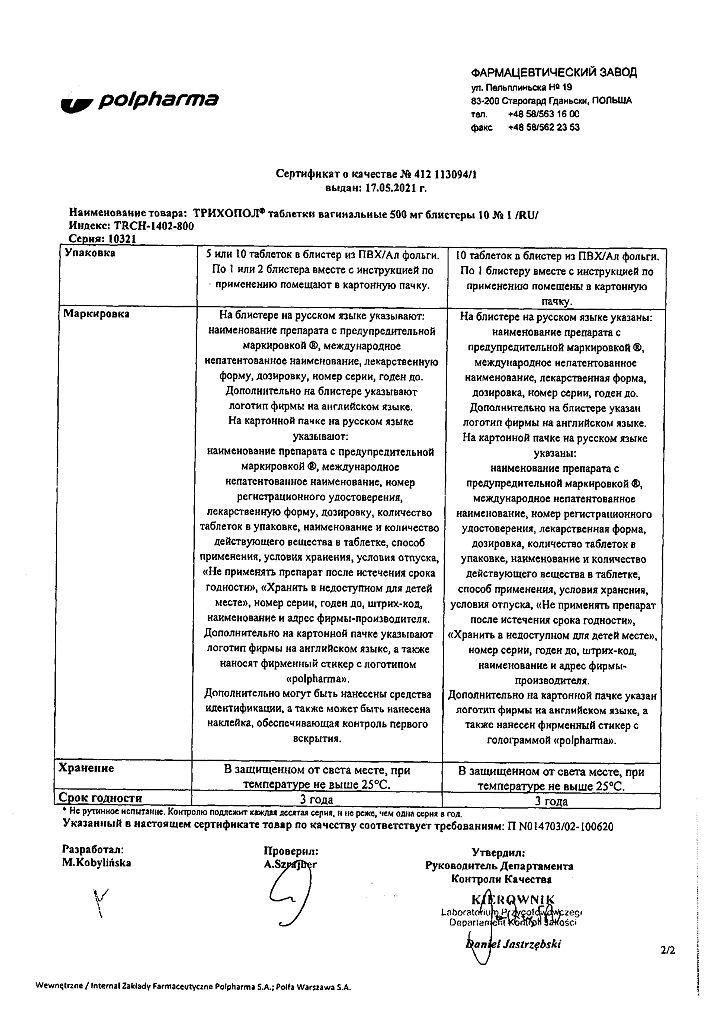
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Polpharma S.A., Poland |
| Medication form | vaginal pills |
| Brand | Polpharma S.A. |
Related products
Gynecology and Obstetrics
Buy Trichopol, vaginal tablets 500 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.