No products in the cart.
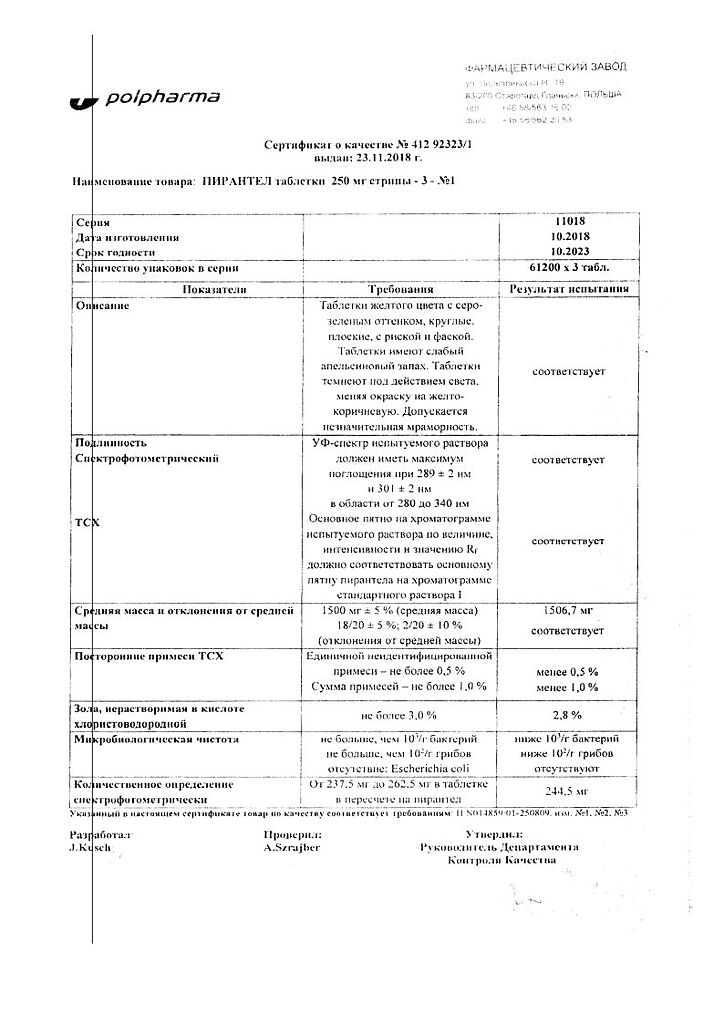
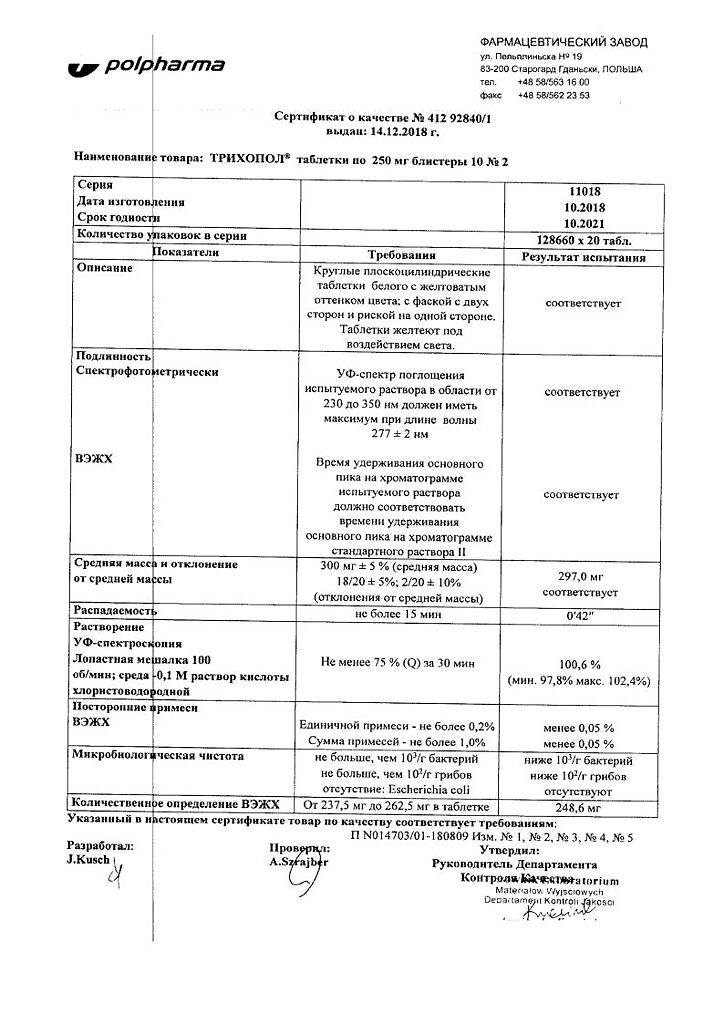
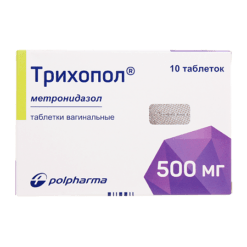
Trichopol, tablets 250 mg 20 pcs
€3.44 €3.13
EAN: 5903060006126
SKU: 34727
Categories: Gynecology and Obstetrics, Medicine, Trichomoniasis and malaria
Description
An antiprotozoal drug with antibacterial activity, a derivative of 5-nitroimidazole. Mechanism of action consists in biochemical reduction of 5-nitrogroup of metronidazole by intracellular transport proteins of anaerobic microorganisms and protozoa. The reduced 5-nitrogroup of metronidazole interacts with the DNA of the microbial cell, inhibiting the synthesis of their nucleic acids, which leads to the death of the microorganisms.
Metronidazole is active against Trichomonas vaginalis, Entamoeba histolytica, Gardnerella vaginalis, Giardia intestinalis, Lamblia spp.Bacteroides spp. (including Bacteroides fragilis, Bacteroides distasonis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides vulgatus), Fusobacterium spp, Veillonella spp., Prevotella spp. (Prevotella bivia, Prevotella buccae, Prevotella disiens); some gram-positive microorganisms (Eubacterium spp., Clostridium spp., Peptococcus spp., Peptostreptococcus spp.) The MPC for these strains is 0.125-6.25 µg/ml.
In combination with amoxicillin it shows activity against Helicobacter pylori (amoxicillin suppresses the development of resistance to metronidazole).
Metronidazole has no bactericidal effect against most bacteria and facultative anaerobes, fungi and viruses. In the presence of mixed flora (aerobes and anaerobes) metronidazole shows synergism with antibiotics effective against common aerobes.
Metronidazole increases the sensitivity of tumors to radiation, causes sensitization to ethanol (disulfiram-like action), stimulates reparative processes.
Indications
Indications
trichomoniasis
intestinal infections
urinary tract infections, female genital infections, gastric ulcers
stomatitis
sepsis, peritonitis.
Pharmacological effect
Pharmacological effect
Antiprotozoal drug with antibacterial activity, a derivative of 5-nitroimidazole. The mechanism of action is the biochemical reduction of the 5-nitro group of metronidazole by intracellular transport proteins of anaerobic microorganisms and protozoa. The reduced 5-nitro group of metronidazole interacts with the DNA of the cell of microorganisms, inhibiting the synthesis of their nucleic acids, which leads to the death of microorganisms.
Metronidazole is active against Trichomonas vaginalis, Entamoeba histolytica, Gardnerella vaginalis, Giardia intestinalis, Lamblia spp.; as well as obligate anaerobes Bacteroides spp. (including Bacteroides fragilis, Bacteroides distasonis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides vulgatus), Fusobacterium spp., Veillonella spp., Prevotella spp. (Prevotella bivia, Prevotella buccae, Prevotella disiens); some gram-positive microorganisms (Eubacterium spp., Clostridium spp., Peptococcus spp., Peptostreptococcus spp.). The MIC for these strains is 0.125-6.25 μg/ml.
In combination with amoxicillin, it is active against Helicobacter pylori (amoxicillin suppresses the development of resistance to metronidazole).
Metronidazole does not have a bactericidal effect against most bacteria and facultative anaerobes, fungi and viruses. In the presence of mixed flora (aerobes and anaerobes), metronidazole exhibits synergism with antibiotics that are effective against common aerobes.
Metronidazole increases the sensitivity of tumors to radiation, causes sensitization to ethanol (disulfiram-like effect), and stimulates reparative processes.
Special instructions
Special instructions
Trichopol® should be prescribed with caution to patients with severe liver failure, because as a result of a slowdown in metabolism, the concentration of metronidazole and its metabolites in plasma increases.
Due to slower excretion, caution is required when choosing the dose of metronidazole in patients with renal impairment.
Caution is required when prescribing Trichopolum to patients with suppression of bone marrow hematopoiesis and central nervous system functions, as well as elderly patients. The appearance of ataxia, dizziness and any other deterioration in the neurological status of patients requires cessation of treatment.
During long-term therapy with metronidazole (more than 10 days), peripheral blood patterns and liver function should be monitored.
With leukopenia, the possibility of continuing treatment depends on the risk of developing an infectious process.
The use of metronidazole should be avoided in patients with porphyria.
Trichopol® can cause immobilization of treponemes, which leads to a false-positive Nelson test.
When treating trichomonas vaginitis in women and trichomonas urethritis in men, it is necessary to abstain from sexual activity. Simultaneous treatment of sexual partners is mandatory. After treatment for trichomoniasis, control tests should be carried out during three consecutive cycles before and after menstruation.
After treatment of giardiasis, if symptoms persist, 3 stool tests should be performed after 3-4 weeks at intervals of several days (in some successfully treated patients, lactose intolerance caused by infestation may persist for several weeks or months, resembling the symptoms of giardiasis).
Patients should refrain from drinking alcohol during therapy with metronidazole, as well as for at least 48 hours after completion of treatment, due to the possibility of developing disulfiram-like reactions: cramping abdominal pain, nausea, vomiting, headache, sudden flushing of the face.
During treatment with metronidazole, urine may become dark or red-brown in color due to the presence of water-soluble dyes.
Use in pediatrics
It is not recommended to use Trichopol® in combination with amoxicillin in children and adolescents under the age of 18 years.
Impact on the ability to drive vehicles and operate machinery
When taking the drug, you should avoid potentially hazardous activities that require increased concentration and speed of psychomotor reactions, especially driving vehicles and servicing machinery.
Active ingredient
Active ingredient
Metronidazole
Composition
Composition
1 tab.:
– metronidazole 250 mg;
excipients:
potato starch,
gelatin,
starch syrup,
magnesium stearate.
Contraindications
Contraindications
diseases of the central nervous system
blood diseases
hypersensitivity to the drug.
Side Effects
Side Effects
From the digestive system: epigastric pain, nausea, vomiting, diarrhea, constipation, intestinal colic, loss of appetite, anorexia, taste disturbance, unpleasant metallic taste in the mouth, dry mouth, glossitis, stomatitis; very rarely – abnormal liver function tests, cholestatic hepatitis, jaundice, pancreatitis.
From the side of the central nervous system: with long-term use – headache, dizziness, impaired coordination of movements, ataxia, peripheral neuropathy, increased excitability, irritability, depression, sleep disturbance, drowsiness, weakness; in some cases – confusion, hallucinations, convulsions; very rarely – encephalopathy.
From the urinary system: dysuria, cystitis, polyuria, urinary incontinence, red-brown coloring of urine.
From the reproductive system: pain in the vagina.
Allergic reactions: skin rash, itching, urticaria, erythema multiforme, nasal congestion, fever.
From the musculoskeletal system: myalgia, arthralgia.
From the hematopoietic system: leukopenia; rarely – agranulocytosis, neutropenia, thrombocytopenia, pancytopenia.
Other: flattening of the T wave on the ECG; extremely rarely – ototoxicity; pustular rashes.
Interaction
Interaction
Metronidazole enhances the effect of warfarin and other coumarin anticoagulants (with this combination, a reduction in the doses of both drugs is required).
Similar to disulfiram, metronidazole causes ethanol intolerance. The simultaneous use of Trichopolum with disulfiram can lead to the development of various neurological symptoms (the interval between the use of these drugs should be at least 2 weeks).
Cimetidine inhibits the metabolism of metronidazole, which may lead to an increase in its concentration in the blood plasma and an increased risk of adverse reactions.
The simultaneous administration of drugs that stimulate the activity of microsomal liver enzymes (phenobarbital, phenytoin) can accelerate the elimination of metronidazole, which leads to a decrease in its concentration in the blood plasma.
In patients receiving long-term treatment with lithium drugs in high doses, when taking metronidazole, the concentration of lithium in the blood plasma may increase and symptoms of intoxication may develop.
It is not recommended to combine metronidazole with non-depolarizing muscle relaxants (vecuronium bromide).
When taking metronidazole and cyclosporine in combination, an increase in the concentration of cyclosporine in the blood plasma may be observed.
Metronidazole reduces the clearance of fluorouracil, which may increase the toxicity of the latter.
Sulfonamides enhance the antimicrobial effect of metronidazole.
Overdose
Overdose
Symptoms: increased side effects, mainly nausea, vomiting and dizziness; in more severe cases, ataxia, paresthesia and convulsions are possible. The lethal dose for humans is unknown.
Treatment: symptomatic and supportive therapy. There is no specific antidote.
Storage conditions
Storage conditions
The drug should be stored in a dry place, protected from light and out of reach of children, at a temperature not exceeding 25°C.
Shelf life
Shelf life
5 years.
Manufacturer
Manufacturer
Polpharma JSC, Poland
Additional information
| Shelf life | 5 years. |
|---|---|
| Conditions of storage | The drug should be stored in a dry place, protected from light and out of reach of children, at a temperature not exceeding 25°C. |
| Manufacturer | Polpharma S.A., Poland |
| Medication form | pills |
| Brand | Polpharma S.A. |
Other forms…
Related products
Gynecology and Obstetrics
Buy Trichopol, tablets 250 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.