No products in the cart.
Triampur Compositum, tablets 12.5mg+25 mg 50 pcs
€15.73 €13.61
Description
Pharmacotherapeutic group: diuretic combination medicine.
ATX code: C03EA01.
Pharmacological properties
Pharmacodynamics
A combination drug, contains hydrochlorthiazide and triamterene. It has diuretic and hypotensive effects.
Hydrochlorothiazide – The mechanism of action of thiazide diuretics (thiazides) is not fully understood. Thiazides block reabsorption of sodium and chlorine ions in the renal tubules. Thus they increase the excretion of sodium and chlorine and therefore the excretion of water from the body.
The diuretic effect of hydrochlorthiazide decreases the volume of circulating fluid, resulting in increased renin activity and plasma aldosterone content. This leads to increased urinary excretion of potassium ions and a decrease in blood potassium content (hypokalemia).
Hydrochlorthiazide also increases the excretion of magnesium ions and decreases the excretion of calcium ions in the urine. Thiazide diuretics decrease renal excretion of uric acid and increase its content in the blood.
Thiazide diuretics also reduce carboangiradase activity by increasing the excretion of bicarbonate ions. But this effect is usually weak and has no effect on urine pH.
In the maximum therapeutic doses, the diuretic/natriuretic effect of all thiazide diuretics is approximately the same. Natriuresis and diuresis occur within 2 hours after administration and reach their maximum after about 4 hours. Duration of diuretic action of hydrochlorothiazide is from 6 to 12 hours.
Hydrochlorothiazide has an antihypertensive effect. Normal arterial pressure is not affected by thiazide diuretics.
Triamterene is a potassium-saving diuretic that reduces the permeability of distal tubule cell membranes to sodium ions and increases their excretion with urine without increasing the excretion of potassium ions. The secretion of potassium ions in the distal tubules is reduced. In combination with hydrochlorothiazide, triamterene is able to reduce hypokalemia caused by thiazide diuretics and increase the diuretic effect of hydrochlorothiazide. Diuretic effect of triamterene after oral administration is observed in 15-20 minutes. The maximum effect is after 2-3 hours, the duration of action is 12 hours.
Pharmacokinetics
Hydrochlorothiazide
absorption and distribution.
Hydrochlorothiazide is incompletely but fairly rapidly absorbed from the gastrointestinal tract. After oral administration in a dose of 100 mg, the maximum concentration of hydrochlorothiazide in blood plasma is reached after 1.5-2.5 hours. At the maximum diuretic activity (about 4 hours after intake), the plasma concentration of hydrochlorothiazide is 2 µg/ml. Binding with plasma proteins is 40%. Hydrochlorothiazide penetrates through the placental barrier and is excreted into breast milk, it does not penetrate through the blood-brain barrier.
Metabolism
Hydrochlorothiazide is not metabolized in humans.
Evolution
The primary route of excretion is through the kidneys (filtration and secretion) unchanged. Approximately 61% of the ingested dose is excreted within 24 hours. In patients with normal renal function, the elimination half-life ranges from 5.6 to 14.8 hours (6.4 hours on average).
Pharmacokinetics in special patient groups
Disordered renal function
In patients with moderate renal impairment, the half-life of hydrochlorothiazide averages 11.5 hours, and in patients with creatinine clearance less than 30 mL/min.- 20.7 hours.
Triamterene is rapidly but not completely (30-70% of the dose taken) absorbed from the gastrointestinal tract. To a moderate extent (67%) it is bound to plasma proteins. Maximum plasma concentration is reached 2-4 hours after administration. The drug is biotransformed in the liver to form both active and inactive metabolites. The normal half-life of the drug is 1.5-2 hours (with anuria – 10 hours), metabolites – up to 12 hours. The main route of excretion of triamterene is through the intestine, the secondary route is by the kidneys.
Indications
Indications
Edema syndrome of various origins (chronic heart failure; nephrotic syndrome, liver cirrhosis);
arterial hypertension.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: diuretic combination drug.
ATX code: C03EA01.
Pharmacological properties
Pharmacodynamics
A combination drug containing hydrochlorothiazide and triamterene. It has a diuretic and hypotensive effect.
Hydrochlorothiazide – the mechanism of action of thiazide diuretics (thiazides) is not fully understood. Thiazides block the reabsorption of sodium and chloride ions in the renal tubules. Thus, they increase the excretion of sodium and chlorine and, consequently, the removal of water from the body.
As a result of the diuretic effect of hydrochlorothiazide, the volume of circulating fluid decreases, resulting in an increase in renin activity and the content of aldosterone in the blood plasma. This leads to an increase in the excretion of potassium ions in the urine and a decrease in potassium levels in the blood (hypokalemia).
Hydrochlorothiazide also increases the excretion of magnesium ions and reduces the excretion of calcium ions in the urine. Thiazide diuretics reduce the excretion of uric acid by the kidneys and increase its content in the blood.
Thiazide diuretics also reduce carbonic anhydrase activity by increasing the excretion of bicarbonate ions. But this effect is usually weak and does not affect the pH of the urine.
At maximum therapeutic doses, the diuretic/natriuretic effect of all thiazide diuretics is approximately the same. Natriuresis and diuresis occur within 2 hours after administration and reach their maximum after approximately 4 hours. The duration of the diuretic effect of hydrochlorothiazide ranges from 6 to 12 hours.
Hydrochlorothiazide has an antihypertensive effect. Thiazide diuretics have no effect on normal blood pressure.
Triamterene is a potassium-sparing diuretic that reduces the permeability of the cell membranes of the distal tubules to sodium ions and increases their excretion in the urine without increasing the excretion of potassium ions. The secretion of potassium ions in the distal tubules decreases. In combination with hydrochlorothiazide, triamterene can reduce hypokalemia caused by thiazide diuretics and enhance the diuretic effect of hydrochlorothiazide. The diuretic effect of triamterene after oral administration is observed after 15-20 minutes. The maximum effect is after 2-3 hours, duration of action is 12 hours.
Pharmacokinetics
Hydrochlorothiazide
Absorption and distribution.
Hydrochlorothiazide is incompletely, but rather quickly absorbed from the gastrointestinal tract. After oral administration at a dose of 100 mg, the maximum concentration of hydrochlorothiazide in the blood plasma is achieved after 1.5-2.5 hours. At maximum diuretic activity (approximately 4 hours after administration), the concentration of hydrochlorothiazide in the blood plasma is 2 mcg/ml. The connection with blood plasma proteins is 40%. Hydrochlorothiazide crosses the placental barrier and is excreted into breast milk, but does not penetrate the blood-brain barrier.
Metabolism
Hydrochlorothiazide is not metabolized in the human body.
Removal
The primary route of excretion is through the kidneys (filtration and secretion) unchanged. Approximately 61% of an oral dose is eliminated within 24 hours. In patients with normal renal function, the half-life ranges from 5.6 to 14.8 hours (average 6.4 hours).
Pharmacokinetics in special groups of patients
Renal dysfunction
In patients with moderate renal impairment, the half-life of hydrochlorothiazide averages 11.5 hours, and in patients with creatinine clearance less than 30 ml/min, it is 20.7 hours.
Triamterene is quickly, but not completely (30-70% of the dose taken) absorbed from the gastrointestinal tract. To a moderate extent (67%) binds to blood plasma proteins. The maximum concentration in blood plasma is achieved 2-4 hours after administration. It undergoes biotransformation in the liver with the formation of both active and inactive metabolites. The half-life of the drug is normally 1.5-2 hours (with anuria – 10 hours), for metabolites – up to 12 hours. The main route of elimination of triamterene is through the intestines, the secondary route is by the kidneys.
Special instructions
Special instructions
Since Triampur Compositum is a combination of two diuretics, during long-term treatment with high doses of its constituent drugs, the possibility of electrolyte imbalance should be considered, especially in patients on a low-salt diet and in patients with conditions affecting electrolyte balance, such as heart failure, kidney and liver disease (cirrhosis).
Fluid and electrolyte imbalances and metabolic disorders (hyperkalemia, hyponatremia, metabolic acidosis and other electrolyte and fluid imbalances)
Periodic monitoring of serum electrolytes is not required. During long-term course treatment with Triampur Compositum®, the potassium concentration should be determined before starting therapy and 3-4 weeks after the end of therapy. If potassium balance is not disturbed, subsequent determination of potassium concentration should be carried out every 4-6 months.
If signs of hyperkalemia appear, taking Triampur Compositum® should be discontinued. In elderly patients and patients simultaneously receiving therapy with digitalis, drugs from the group of glucocorticosteroids, laxatives, or intravenous infusions, strict monitoring of water and electrolyte balance is necessary.
Kidney failure
Thiazide diuretics should be used with caution in patients with impaired renal function. The dosage should be reduced in patients with renal failure (creatinine clearance>30 ml/min). Thiazide diuretics are ineffective against progressive renal dysfunction (creatinine clearance < 30 ml/min), and may also worsen impaired renal function or may cause azotemia. In patients with impaired renal function, periodic monitoring of serum urea and creatinine clearance is necessary.
Liver failure
Triamterene + hydrochlorothiazide, like other thiazide diuretics, should be used with caution in patients with severe hepatic impairment or progressive liver disease, since even small changes in fluid and electrolyte balance can cause hepatic coma.
Hydrochlorothiazide
Renal dysfunction
In patients with impaired renal function, hydrochlorothiazide may cause azotemia. In case of renal failure, accumulation of hydrochlorothiazide is possible.
In patients with reduced renal function, periodic monitoring of creatinine clearance is necessary. If renal dysfunction progresses and/or oliguria (anuria) occurs, hydrochlorothiazide should be discontinued.
Liver dysfunction
When using thiazide diuretics in patients with impaired liver function, hepatic encephalopathy may develop. In patients with severe liver failure or hepatic encephalopathy, the use of thiazides is contraindicated. In patients with mild to moderate hepatic impairment and/or progressive liver disease, hydrochlorothiazide should be used with caution, since even slight changes in fluid and electrolyte balance and serum ammonium accumulation can cause hepatic coma. If symptoms of encephalopathy occur, diuretics should be discontinued immediately.
Water-electrolyte balance and metabolic disorders
Thiazide diuretics (including hydrochlorothiazide) can cause a decrease in the volume of circulating fluid (hypovolemia) and disturbances in water and electrolyte balance (including hypokalemia, hyponatremia, hypochloremic alkalosis). Clinical symptoms of fluid and electrolyte imbalance include dry mouth, thirst, weakness, lethargy, fatigue, drowsiness, restlessness, muscle pain or cramps, muscle weakness, marked decrease in blood pressure, oliguria, tachycardia, arrhythmia and gastrointestinal disorders (such as nausea and vomiting). In patients receiving hydrochlorothiazide therapy (especially with long-term course treatment), clinical symptoms of fluid and electrolyte imbalance should be identified and blood electrolyte levels should be regularly monitored.
Sodium
All diuretics can cause hyponatremia, sometimes leading to severe complications. Hyponatremia and hypovolemia can lead to dehydration and orthostatic hypotension. A concomitant decrease in the level of chlorine ions in the blood plasma can lead to secondary compensatory metabolic alkalosis, but the frequency and severity of this effect are insignificant. It is recommended to determine the content of sodium ions in the blood plasma before starting treatment and regularly monitor this indicator while taking hydrochlorothiazide.
Potassium
When using thiazide and thiazide-like diuretics, there is a risk of a sharp decrease in the potassium content in the blood plasma and the development of hypokalemia (potassium concentration less than 3.4 mmol/l). Hypokalemia increases the risk of developing heart rhythm disturbances (including severe arrhythmias) and enhances the toxic effect of cardiac glycosides. In addition, hypokalemia (as well as bradycardia) is a condition that contributes to the development of polymorphic ventricular tachycardia of the “pirouette” type, which can be fatal.
Hypokalemia poses the greatest danger to the following groups of patients: 1) elderly people, 2) patients simultaneously receiving therapy with antiarrhythmic and non-antiarrhythmic drugs that can cause polymorphic ventricular tachycardia of the “pirouette” type or increase the duration of the QT interval on the ECG, 3) patients with impaired liver function, coronary heart disease, chronic heart failure. In addition, patients with an increased QT interval are at increased risk. It does not matter whether this increase is caused by congenital causes or the effect of drugs.
In all the cases described above, it is necessary to avoid the risk of developing hypokalemia and regularly monitor the potassium content in the blood plasma. The first measurement of the content of potassium ions in the blood must be carried out within the first week from the start of treatment. If hypokalemia occurs, appropriate treatment should be prescribed. Hypokalemia can be corrected by using potassium-containing medications or eating foods rich in potassium (dried fruits, fruits, vegetables).
Calcium
Thiazide diuretics may reduce the excretion of calcium ions by the kidneys, leading to a slight and temporary increase in plasma calcium levels. In some patients, with long-term use of thiazide diuretics, pathological changes in the parathyroid glands were observed with hypercalcemia and hyperphosphatemia, but without the typical complications of hyperparathyroidism (nephrolithiasis, decreased bone mineral density, peptic ulcer).
Severe hypercalcemia may be a manifestation of previously undiagnosed hyperparathyroidism.
Due to their effect on calcium metabolism, thiazides may affect laboratory parameters of parathyroid function. Thiazide diuretics (including hydrochlorothiazide) should be discontinued before testing parathyroid function.
Magnesium
Thiazides have been found to increase renal excretion of magnesium, which can lead to hypomagnesemia. The clinical significance of hypomagnesemia remains unclear.
Glucose
Treatment with thiazide diuretics may impair glucose tolerance. When using hydrochlorothiazide in patients with manifest or latent diabetes mellitus, it is necessary to regularly monitor the concentration of glucose in the blood. Dosage adjustment of hypoglycemic medications may be required.
Uric acid
In patients with gout, the frequency of attacks may increase or the course of gout may worsen. Careful monitoring of patients with gout and impaired uric acid metabolism (hyperuricemia) is necessary.
Lipids
When using hydrochlorothiazide, the concentration of cholesterol and triglycerides in the blood plasma may increase.
Acute myopia/secondary angle-closure glaucoma
Symptoms include: sudden loss of vision or eye pain, usually occurring within hours to weeks of starting hydrochlorothiazide therapy. If left untreated, acute angle-closure glaucoma can lead to irreversible vision loss. If symptoms appear, you should stop taking hydrochlorothiazide as soon as possible. If intraocular pressure remains uncontrolled, emergency medical treatment or surgery may be required.
Risk factors for the development of acute angle-closure glaucoma are: a history of an allergic reaction to sulfonamides or penicillin.
Immune system disorders
There are reports that thiazide diuretics (including hydrochlorothiazide) may cause exacerbation or progression of systemic lupus erythematosus, as well as lupus-like reactions. In patients receiving thiazide diuretics, hypersensitivity reactions may occur even in the absence of a history of allergic reactions or bronchial asthma.
Photosensitivity
There is information about cases of the development of photosensitivity reactions when taking thiazide diuretics. If photosensitivity occurs while taking
hydrochlorothiazide treatment should be discontinued. If continued use of a diuretic is necessary, the skin should be protected from exposure to sunlight or
artificial ultraviolet rays.
Non-melanoma skin cancer
Two pharmacoepidemiological studies using data from the Danish National Cancer Registry demonstrated an association between hydrochlorothiazide use and an increased risk of non-melanoma skin cancer (NMSC) basal cell carcinoma and squamous cell carcinoma. The risk of developing NMSC increased with increasing total (cumulative) dose of hydrochlorothiazide. A possible mechanism for the development of NMSC is the photosensitizing effect of hydrochlorothiazide.
Patients taking hydrochlorothiazide as monotherapy or in combination with other drugs should be aware of the risk of developing
NMRK. Such patients are advised to regularly examine the skin in order to identify any new suspicious lesions, as well as changes in existing ones.
skin lesions.
Any suspicious skin changes should be reported to your doctor immediately. Suspicious areas of skin should be examined by a specialist. For clarification
diagnosis may require histological examination of skin biopsies.
To minimize the risk of developing NMSC, patients should be advised to follow preventive measures, such as limiting exposure to sunlight and UV rays, and using appropriate protective equipment.
In patients with a history of non-melanoma skin cancer, it is recommended to reconsider the use of hydrochlorothiazide.
Choroidal effusion, acute myopia and secondary angle-closure glaucoma
Sulfonamides or their derivatives can cause an idiosyncratic reaction leading to choroidal effusion with loss of visual fields, transient myopia and acute angle-closure glaucoma. Symptoms include sudden loss of vision or pain in the eye and usually occur within hours to weeks of starting the drug. If not treated promptly, acute angle-closure glaucoma can lead to irreversible vision loss. When the first symptoms appear, you must immediately stop using the drug. If elevated intraocular pressure persists, emergency medical or surgical treatment may be required.
Risk factors for acute angle-closure glaucoma include a history of allergic reactions to sulfonamides or penicillin.
Alcohol
During treatment with hydrochlorothiazide, it is not recommended to drink alcoholic beverages, because ethanol enhances the antihypertensive effect of thiazide diuretics.
Other
In patients with severe atherosclerosis of the cerebral and coronary arteries, hydrochlorothiazide should be used with extreme caution.
Thiazide diuretics can reduce the amount of iodine bound to plasma proteins without causing signs of thyroid dysfunction.
Triamterene
Potassium
Due to the potassium-sparing effect of triamterene, hypokalemia with the use of Triampur compositum® is rare, but may occur in some cases when the triamterene component does not adequately compensate for the loss of potassium due to the thiazide component or in a certain clinical picture, or with concomitant use of drugs that can cause hypokalemia, such as glucocorticosteroids, corticotropin, amphotericin B and carbenoxolone.
Uric acid
In patients, serum uric acid concentrations may be increased and clinical signs of gout may appear. Periodic monitoring of serum uric acid levels is necessary during drug therapy.
Folic acid
If folic acid deficiency is suspected (for example, with liver cirrhosis and chronic alcohol use), regular monitoring of blood counts is necessary, since triamterene, being a weak folate antagonist, can contribute to the development of megaloblastic anemia.
Carbohydrate metabolism
Taking the drug may impair glucose tolerance; therefore, in patients with manifest or latent diabetes mellitus, it is necessary to regularly monitor the concentration of glucose in the blood. Dosage adjustments of hypoglycemic agents and insulin may be required.
Metabolic acidosis
Triamterene can lead to increased excretion of alkaline blood components, which can lead to the development of metabolic acidosis.
Urolithiasis
The use of triamterene can lead to the formation of kidney stones, which in the kidneys consist entirely or partially of triamterene and its metabolites.
Caution is advised when treating patients with a history of urolithiasis, since triamterene may promote stone enlargement.
Other:
When using triamterene, it must be borne in mind that urine may turn blue, especially when using high doses.
Impact on the ability to drive vehicles and machinery
Due to the possible excessive decrease in blood pressure (which may manifest itself as a slowdown in the speed of reactions), especially at the beginning of treatment, care must be taken when driving vehicles and machinery.
Active ingredient
Active ingredient
Hydrochlorothiazide, Triamterene
Composition
Composition
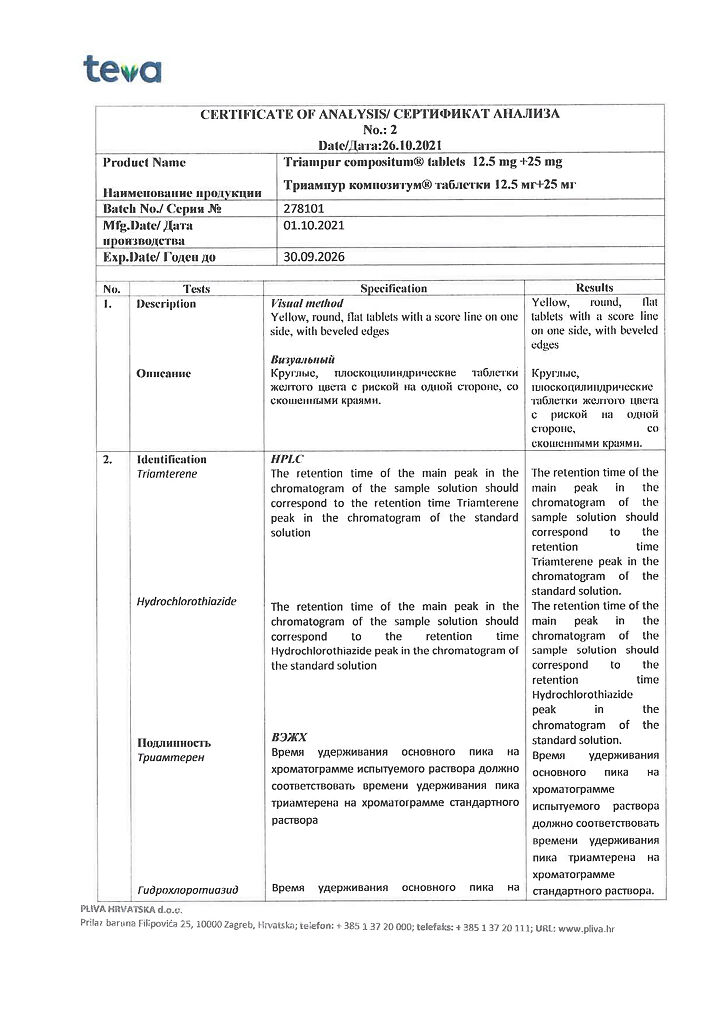
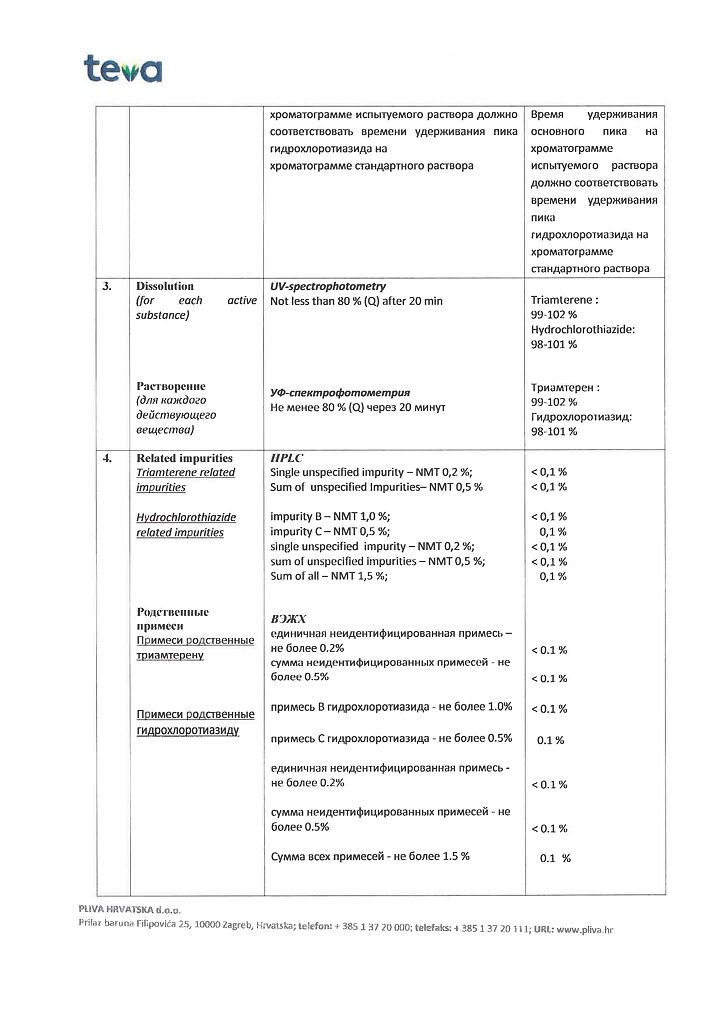
1 tablet contains:
Pregnancy
Pregnancy
Triampur compositum® is contraindicated for use during pregnancy and breastfeeding.
Hydrochlorothiazide
Pregnancy
There is limited experience with the use of hydrochlorothiazide during pregnancy (especially in the first trimester). Preclinical data regarding safety are insufficient.
Hydrochlorothiazide penetrates the placental barrier and is detected in umbilical cord blood. Taking into account the mechanism of pharmacological action of hydrochlorothiazide, its use in the second and third trimesters of pregnancy can disrupt fetoplacental perfusion and lead to the development of complications such as jaundice, water-electrolyte imbalance and thrombocytopenia in the fetus and newborn. Cases of the development of thrombocytopenia in newborns whose mothers received thiazide diuretics have been described.
The use of hydrochlorothiazide in the first trimester of pregnancy is contraindicated. In the second and third trimesters of pregnancy, hydrochlorothiazide can be used only if absolutely necessary, when the benefit to the mother outweighs the potential risk to the fetus and/or child.
Hydrochlorothiazide should not be used to treat gestosis in the second half of pregnancy (edema, arterial hypertension or preeclampsia), because it increases the risk of decreased circulating blood volume and placental hypoperfusion, but does not have a beneficial effect on the course of these pregnancy complications. Diuretics do not prevent the development of gestosis.
Breastfeeding period
Hydrochlorothiazide passes into breast milk, and therefore its use during breastfeeding is contraindicated. If the use of hydrochlorothiazide during lactation is absolutely necessary, then breastfeeding should be discontinued.
Triamterene
Triamterene crosses the placenta and is excreted in breast milk. Because triamterene inhibits folic acid metabolism, adverse effects cannot be excluded.
Contraindications
Contraindications
– hypersensitivity to hydrochlorothiazide, other sulfonamide derivatives, triamterene or any of the excipients;
– severe renal failure (creatinine clearance (CC) less than 30 ml/min) or progressive renal failure;
– acute glomerulonephritis;
– anuria;
– severe liver failure or hepatic encephalopathy (risk of developing hepatic coma);
– hyperkalemia (˃ 5.5 mmol/l);
– insufficiency of the adrenal cortex (including Addison’s disease);
– refractory hypokalemia, hyponatremia, hypercalcemia;
– pregnancy and breastfeeding period;
– children under 18 years of age;
– rare hereditary galactose intolerance, lactase deficiency, glucose-galactose malabsorption syndrome (the drug contains lactose monohydrate)
With caution
– Arterial hypotension
– Renal dysfunction
– Mild to moderate liver dysfunction, progressive liver disease
– Hypokalemia
– Hyponatremia
– Hypercalcemia
– Concomitant use of drugs that can cause polymorphic ventricular tachycardia of the “pirouette” type or increase the duration of the QT interval on the ECG
– Simultaneous use of lithium preparations, drugs that can cause hypokalemia or hyperkalemia, cardiac glycosides
– History of allergic reactions to penicillin
– History of allergic reactions to sulfonamides
– Hyperparathyroidism
– Diabetes mellitus
– Hyperuricemia, gout
– Systemic lupus erythematosus
– Coronary heart disease, severe atherosclerosis of the coronary or cerebral arteries
– Folic acid deficiency (for example, in liver cirrhosis, chronic alcoholism)
– Metabolic acidosis
– Urolithiasis (including history)
– History of non-melanoma skin cancer (NSC) (see section “Special instructions”)
– Old age
Side Effects
Side Effects
Side effects of the drug Triampur compositum® are usually dose-dependent, most of them are moderate or mild and disappear when the dose is reduced or the drug is discontinued.
Adverse reactions when using the drug are distributed according to systemic organ classes, indicating the frequency of their occurrence in accordance with the following scale: very often (≥1/10), often (≥1/100, ˂ 1/10), infrequently (≥1/1000, ˂ 1/100), rarely (≥1/10000, ˂ 1/1000), very rarely (˂ 1/10000), the frequency is unknown (cannot be estimated from the available data) and is presented in the table.
Table 1. Side effects (or Adverse reactions)
Systemic organ
Class
Unwanted
reaction
Frequency of occurrence
Hydrochlorothiazide + Triamterene
Hydrochloro-
thiazide
Triamterene
Neoplasms, benign, malignant and unspecified (including cysts and polyps)1)
Non-melanoma
skin cancer (basal-
cell carcinoma and squamous cell carcinoma)
frequency unknown
frequency unknown
–
Blood and lymphatic system disorders2)
thrombocytopenic purpura,
neutropenia, leukopenia, thrombocytopenia, agranulocytosis, aplastic anemia
frequency unknown
–
frequency unknown
rarely
Immune system disorders
anaphylactic reactions,
Steven-Johnson syndrome,
Lyell’s syndrome, urticaria, itching, photosensitivity,
vasculitis, exacerbation of existing systemic lupus erythematosus, erythema multiforme
frequency unknown
frequency unknown
–
Metabolic and nutritional disorders
hyperkalemia,
hyperglycemia,
hyperuricemia incl. with the development of a gout attack,
hypercalcemia,
hypokalemia,
hyponatremia
hypochloremia
metabolic acidosis,
dyslipidemia
frequency unknown
frequency unknown
–
Nervous system disorders
parasthesia,
dizziness,
weakness,
fatigue,
depression,
headache,
muscle spasms and cramps
frequency unknown
frequency unknown
–
Mental disorders
decreased libido
frequency unknown
–
–
Visual disorders
xanthopsia, temporary blurred vision, choroidal effusion
frequency unknown
–
–
Cardiac disorders3)
arrhythmias,
orthostatic
hypotension
frequency unknown
frequency unknown
–
Vascular disorders
hypotension
frequency unknown
frequency unknown
–
Respiratory, thoracic and mediastinal disorders
allergic pneumonitis, allergic pulmonary edema, respiratory failure;
frequency unknown
–
–
Gastrointestinal disorders 4
pancreatitis,
dry mouth,
sialadenitis,
loss of appetite,
gastrointestinal disorders,
nausea,
vomit,
stomach pain,
constipation or diarrhea
frequency unknown
frequency unknown
–
Disorders of the liver and biliary tract
Intrahepatic cholestatic jaundice,
increased activity of liver enzymes
frequency unknown
frequency unknown
–
Skin and subcutaneous tissue disorders
photosensitivity
skin rash, urticaria, purpura
frequency unknown
rarely
frequency unknown
–
Musculoskeletal and connective tissue disorders
Systemic lupus erythematosus
muscle weakness,
muscle spasms and cramps
very rarely
frequency unknown
–
–
Renal and urinary tract disorders5
acute renal failure,
frequent urination,
polyuria, nocturia, increased levels of urea and creatinine in the blood serum, abnormal urine sediment, interstitial nephritis, glucosuria,
formation of kidney stones
–
–
very rarely
frequency unknown
General disorders and reactions at the injection site
weakness
–
frequency unknown
–
Laboratory and instrumental data
decline
glomerular filtration rate (expressed as increased levels of urea nitrogen, creatinine and/or uric acid in the blood), deviations in levels
frequency unknown
–
–
1) Two pharmacoepidemiological studies using data from the Danish National Cancer Registry demonstrated an association between hydrochlorothiazide use and an increased risk of non-melanoma skin cancer (NMSC) basal cell carcinoma and squamous cell carcinoma. The risk of developing NMSC increased with increasing total (cumulative) dose of hydrochlorothiazide. A possible mechanism for the development of NMSC is the photosensitizing effect of hydrochlorothiazide.
2) In patients with latent folic acid deficiency (alcoholic cirrhosis, pregnancy), triamterene can cause megaloblastic and/or macrocytic anemia.
In vitro studies have shown that triamterene inhibits the utilization of folic acid by dividing cells, probably due to competition between triamterene and folic acid or its natural reducing product, dihydrofolic acid, for the enzyme dihydrofolate reductase.
3) Arrhythmia and orthostatic hypotension can be aggravated by alcohol, barbiturates and drugs.
4) Particular attention should be paid to the fact that gagging and vomiting can also be signs of electrolyte imbalance and pancreatitis
5)) The use of triamterene in high doses can lead to the development of kidney stones. These kidney stones are composed entirely or partially of triamterene and its metabolites. With long-term therapy, glomerular filtration decreases in many patients, especially in patients whose renal function was impaired before treatment.
Interaction
Interaction
Hydrochlorothiazide + triamterene
Potassium supplements and/or potassium-sparing diuretics (spironolactone, amiloride) should not be used concomitantly with Triampur compositum due to the risk of developing potentially serious hyperkalemia.
Triampur compositum® has a hypotensive effect. If the drug is prescribed while continuing existing antihypertensive therapy, it is advisable to reduce the dose of Triampur compositum® by half. Conversely, if the patient is already taking Triampur compositum®, it is advisable to halve the dose of the new antihypertensive drug (for example, a beta blocker) added to Triampur compositum® therapy.
Disopyramide, when used in combination with Triampur compositum®, increases the adverse effects of hyperkalemia and can cause moderate hyperkalemia to become life-threatening.
In patients with diabetes, insulin requirements may change.
The use of ACE inhibitors may lead to increased serum potassium levels. When using this type of drug simultaneously with Triampur compositum®, caution should be exercised.
Lithium preparations should generally not be combined with diuretics because diuretics reduce the excretion of lithium through the kidneys and thereby increase the risk of lithium toxicity.
In recent years, interactions between diuretics and nonsteroidal anti-inflammatory drugs (NSAIDs) have been reported in the literature, which may lead to acute decline in renal function. This interaction has also been described for triamterene and indomethacin. Therefore, caution is advised in patients receiving these drugs concomitantly.
Caution should be exercised when taking Triampur compositum® together with drugs that can cause hypokalemia, such as corticosteroids, corticotropin, amphotericin B or carbenoxolone. In patients with hypokalemia, the myocardial effects of cardiac glycosides and non-depolarizing neuromuscular blockers may be enhanced; previously tolerated doses of digitalis preparations may cause symptoms of intoxication.
Concomitant use with chlorpropamide may increase the risk of developing severe hyponatremia.
Hydrochlorothiazide
Not recommended drug combinations
Lithium preparations
With the simultaneous use of hydrochlorothiazide and lithium preparations, the renal clearance of lithium is reduced, which can lead to an increase in the concentration of lithium in the blood plasma and an increase in its toxicity. If necessary, simultaneous use
hydrochlorothiazide, the dose of lithium preparations should be carefully selected, the concentration of lithium in the blood plasma should be regularly monitored and the dose of the drug should be adjusted accordingly.
Combinations of drugs requiring special attention
Drugs that can cause polymorphic ventricular tachycardia of the “pirouette” type
Hydrochlorothiazide should be used with extreme caution concomitantly with drugs such as:
antiarrhythmic drugs class IA (quinidine, hydroquinidine, disopyramide, procainamide) and class 1C (flecainide);
class III antiarrhythmic drugs (dofetilide, ibutilide, bretylium tosylate), sotalol, dronedarone, amiodarone;
other (non-antiarrhythmic) medicines such as:
– neuroleptics: phenothiazines (chlorpromazine, cyamemazine, levomepromazine, thioridazine, trifluoperazine, fluphenazine), benzamides (amisulpride, sultopride, sulpride, tiapride), butyrophenones (droperidol, haloperidol); pimozide, sertindole;
– antidepressants: tricyclic antidepressants, selective serotonin reuptake inhibitors (citalopram, escitalopram);
– antibacterial agents: fluoroquinolones (levofloxacin, moxifloxacin, sparfloxacin, ciprofloxacin); macrolides (erythromycin for intravenous administration, azithromycin, clarithromycin, roxithromycin, spiramycin), co-trimoxazole;
– antifungals: azoles (voriconazole, itraconazole, ketoconazole, fluconazole);
– antimalarials (quinine, chloroquine, mefloquine, halofantrine, lumefantrine);
– antiprotozoal drugs (pentamidine for parenteral administration);
– antianginal drugs (ranolazine, bepridil);
– antitumor agents (vandetanib, arsenic trioxide, oxaliplatin, tacrolimus);
– antiemetics (domperidone, ondansetron);
– drugs affecting gastrointestinal motility (cisapride);
– antihistamines (astemizole, terfenadine, mizolastine);
– other medicines (anagrelide, vasopressin, difemanil methyl sulfate, ketanserin, probucol, propofol, sevoflurane, terlipressin, terodiline, cilostazol);
– due to an increased risk of ventricular arrhythmias, especially polymorphic ventricular tachycardia of the “pirouette” type (risk factor – hypokalemia).
The potassium level in the blood plasma should be determined and, if necessary, adjusted before starting combination therapy with hydrochlorothiazide with the above drugs.
It is necessary to monitor the patient’s clinical condition, blood plasma electrolyte levels and ECG parameters. In patients with hypokalemia, it is necessary to use drugs that do not cause polymorphic ventricular tachycardia of the “pirouette” type.
Medicines that can prolong the QT interval
The simultaneous use of hydrochlorothiazide with drugs that can prolong the QT interval should be based on a careful assessment for each patient of the expected benefit versus the potential risk (possible increased risk of developing torsade de pointes (TdP). When using such combinations, it is necessary to regularly record an ECG (to detect prolongation of the QT interval), as well as monitor potassium levels in the blood.
Drugs that can cause hypokalemia: amphotericin B (with intravenous administration), gluco- and mineralocorticosteroids (with systemic use), tetracosactide (ACTH), glycyrrhizic acid (carbenoxolone, preparations containing licorice root), laxatives that stimulate intestinal motility.
Increased risk of hypokalemia when used simultaneously with hydrochlorothiazide (additive effect).
Regular monitoring of the potassium content in the blood plasma is necessary, and, if necessary, its correction. During therapy with hydrochlorothiazide, it is recommended to use laxatives that do not stimulate intestinal motility.
Cardiac glycosides
Hypokalemia and hypomagnesemia caused by the action of thiazide diuretics increase the toxicity of cardiac glycosides. When using hydrochlorothiazide and cardiac glycosides simultaneously, you should regularly monitor the concentration of potassium in the blood plasma, ECG readings, and, if necessary, adjust therapy.
Drug combinations requiring attention
Other antihypertensive drugs
Potentiation of antihypertensive action. It may be necessary to adjust the dose of concomitantly prescribed antihypertensive drugs.
Ethanol, barbiturates, antipsychotics (neuroleptics), antidepressants, anxiolytics, narcotic analgesics and general anesthesia
It is possible to enhance the antihypertensive effect of hydrochlorothiazide and potentiate orthostatic hypotension (additive effect).
Non-depolarizing muscle relaxants (eg, tubocurarine)
The effect of non-depolarizing muscle relaxants may be enhanced.
Adrenergic agonists (pressor amines)
Hydrochlorothiazide may reduce the effect of adrenergic agonists such as epinephrine (adrenaline) and norepinephrine (norepinephrine).
Nonsteroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors and high doses of acetylsalicylic acid (>3 g/day)
NSAIDs may reduce the diuretic and antihypertensive effects of hydrochlorothiazide. With simultaneous use, there is a risk of developing acute renal failure due to a decrease in glomerular filtration rate. Hydrochlorothiazide may enhance the toxic effects of high doses of salicylates on the central nervous system.
Oral hypoglycemic agents and insulin
Thiazide diuretics affect glucose tolerance (hyperglycemia may develop) and reduce the effectiveness of hypoglycemic agents (dose adjustment of hypoglycemic agents may be required). Hydrochlorothiazide and metformin should be used together with caution due to the risk of lactic acidosis due to renal impairment caused by hydrochlorothiazide.
Beta blockers, diazoxide
Concomitant use of thiazide diuretics (including hydrochlorothiazide), beta-blockers or diazoxide may increase the risk of hyperglycemia
Medicines used to treat gout (probenecid, sulfinpyrazone, allopurinol)
Dose adjustment of uricosuric drugs may be required as hydrochlorothiazide increases serum uric acid concentrations. Thiazide diuretics may increase the incidence of hypersensitivity reactions to allopurinol.
Amantadine
Thiazide diuretics (including hydrochlorothiazide) may reduce the clearance of amantadine, lead to increased plasma concentrations of amantadine and increase the risk of adverse effects.
Anticholinergic drugs (cholinergic blockers)
Anticholinergic drugs (eg, atropine, biperiden) increase the bioavailability of thiazide diuretics by reducing gastrointestinal motility and the rate of gastric emptying.
Cytotoxic (antitumor) drugs
Thiazide diuretics reduce the renal excretion of cytotoxic drugs (for example, cyclophosphamide and methotrexate) and potentiate their myelosuppressive effects.
Methyldopa
Cases of hemolytic anemia have been described with the simultaneous use of hydrochlorothiazide and methyldopa.
Antiepileptic drugs (carbamazepine, oxcarbazepine, topiramate)
Risk of developing symptomatic hyponatremia. When using hydrochlorothiazide and carbamazepine simultaneously, it is necessary to monitor the patient’s condition and monitor the sodium level in the blood serum. When using hydrochlorothiazide and topiramate simultaneously, the level of topiramate in the blood serum should also be monitored, and if necessary, prescribe potassium supplements or adjust the dose of topiramate.
Selective serotonin reuptake inhibitors
When used simultaneously with thiazide diuretics, hyponatremia may be potentiated. Monitoring of sodium levels in blood plasma is necessary.
Cyclosporine
With simultaneous use of thiazide diuretics and cyclosporine, the risk of developing hyperuricemia and exacerbation of gout increases.
Oral anticoagulants
Thiazide diuretics may reduce the effect of oral anticoagulants
Iodinated contrast agents
Dehydration while taking thiazide diuretics increases the risk of developing acute renal failure, especially when using high doses of iodinated contrast agents. Before using iodinated contrast agents, it is necessary to compensate for fluid loss.
Calcium preparations
With simultaneous use, it is possible to increase the calcium level in the blood and develop hypercalcemia due to a decrease in the excretion of calcium ions by the kidneys. If simultaneous administration of calcium-containing drugs is necessary, the calcium level in the blood plasma should be monitored and the dose of calcium supplements should be adjusted. It is also necessary to combine with caution the simultaneous administration of the drug Triampur compositum® with vitamin D, since hydrochlorothiazide reduces the excretion of calcium in the urine and increases its concentration in the blood, as indicated above
Anion exchange resins (cholestyramine and colestipol)
Anion exchange resins reduce the absorption of hydrochlorothiazide. Single doses of cholestyramine and colestipol reduce the absorption of hydrochlorothiazide in
gastrointestinal tract by 85% and 43%, respectively.
Triamterene
Nonsteroidal anti-inflammatory drugs (NSAIDs)
When used simultaneously with NSAIDs, a decrease in the antihypertensive effect and progression of renal failure are possible.
Drugs that increase potassium levels in the blood
There is a risk of developing hyperkalemia when used simultaneously with spironolactone, potassium salts, and ACE inhibitors.
Amantadine
When used simultaneously with amantadine, an increase in the concentration of amantadine is observed, and there is a risk of toxicity.
Trimethoprim
There may be a risk of developing severe hyponatremia when used concomitantly with trimethoprim.
Overdose
Overdose
Hydrochlorothiazide
Symptoms: marked decrease in blood pressure, polyuria, nausea, vomiting, weakness, confusion, fever, redness of the facial skin, increased neuromuscular excitability, cardiac arrhythmias, ECG changes, convulsions leading to coma. Most of these symptoms are caused by hyperkalemia, electrolyte imbalance, dehydration, and changes in acid-base balance.
Treatment: symptomatic, there is no specific antidote. Immediate gastric lavage and administration of activated carbon are indicated to reduce absorption. In case of arterial hypotension, circulating blood volume (CBV) should be normalized and vasopressor drugs should be administered. Monitoring and correction of water, electrolyte and metabolic balance is recommended. It has not been established to what extent hydrochlorothiazide can be removed from the body by hemodialysis.
Triamterene
No information available
Storage conditions
Storage conditions
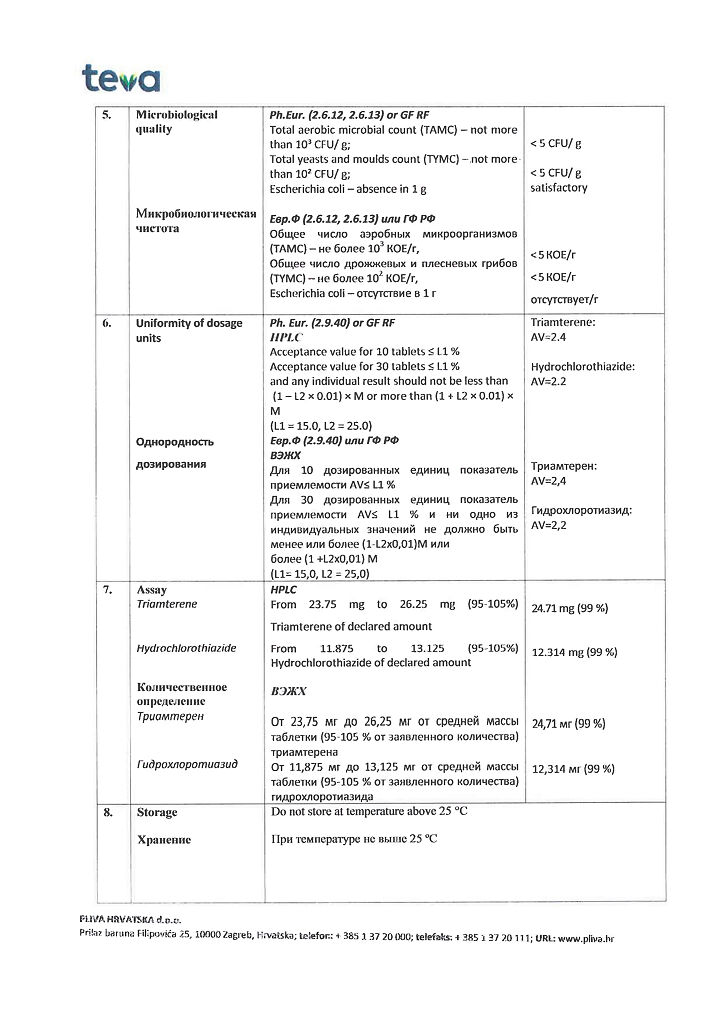
Store at a temperature not exceeding 25°C.
Keep out of the reach of children.
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Pliva Hrvatska d.o.o., Croatia
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Pliva Hrvatska d.o.o., Croatia |
| Medication form | pills |
| Brand | Pliva Hrvatska d.o.o. |
Related products
Buy Triampur Compositum, tablets 12.5mg+25 mg 50 pcs with delivery to USA, UK, Europe and over 120 other countries.