No products in the cart.
Taufon, eye drops 4% 10 ml
€4.25 €3.78
Description
Pharmacotherapeutic group: metabolic agent.
The ATX code: SO1XA.
Pharmacological properties: Taurine is a sulfur-containing amino acid formed in the body during conversion of cysteine. It stimulates the processes of repair and regeneration in diseases of dystrophic nature and diseases accompanied by acute metabolic disorder of eye tissues.
Promotes normalization of cell membrane functions, activation of energy and metabolic processes, preservation of the electrolyte composition of the cytoplasm due to K+ and Ca2+ accumulation, improvement of nerve impulse conducting conditions.
Pharmacokinetics:
In local administration systemic absorption is low.
Indications
Indications
The drug is prescribed to adults for:
– Corneal dystrophies;
– Senile, traumatic, radiation and other types of cataracts;
– Corneal injuries (as a stimulator of reparative processes);
– Primary open-angle glaucoma in combination with β-blockers (to improve the outflow of aqueous humor).
For all indications, the drug is used as part of complex therapy.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: metabolic agent.
ATX code: SO1XA.
Pharmacological properties: Taurine is a sulfur-containing amino acid formed in the body during the conversion of cysteine. Stimulates repair and regeneration processes in diseases of a dystrophic nature and diseases accompanied by a sharp disturbance in the metabolism of eye tissue.
Helps normalize the functions of cell membranes, activate energy and metabolic processes, preserve the electrolyte composition of the cytoplasm due to the accumulation of K+ and Ca2+, and improve the conditions for nerve impulse conduction.
Pharmacokinetics:
When applied topically, systemic absorption is low.
Active ingredient
Active ingredient
Taurine
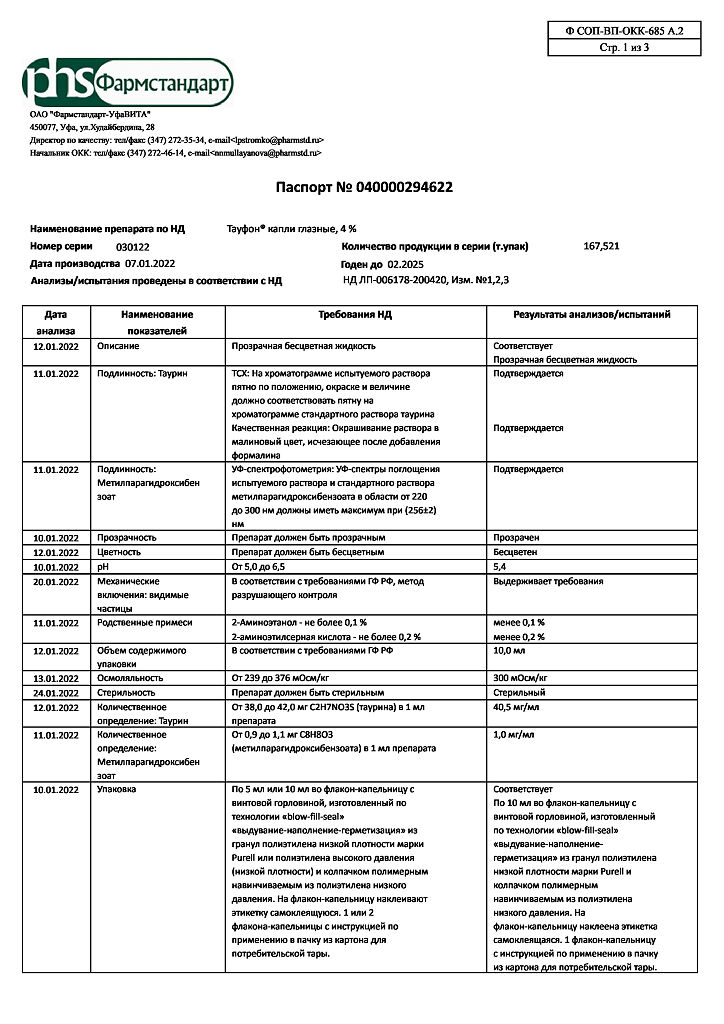
Composition
Composition
1 ml of solution contains:
Active substance:
taurine – 40 mg
Excipients:
methyl parahydroxybenzoate (methylparaben) – 1 mg
1 M sodium hydroxide solution – up to pH 5.0 – 6.5,
Water for injection – up to 1 ml
Pregnancy
Pregnancy
Sufficient
There is no experience with the use of the drug during pregnancy or breastfeeding.
It is possible to use Taufon for the treatment of pregnant and nursing mothers according to
prescribed by the attending physician if the expected therapeutic effect exceeds the risk
development of possible side effects.
Contraindications
Contraindications
Hypersensitivity to taurine,
children under 18 years of age.
Side Effects
Side Effects
Allergic reactions may occur.
Interaction
Interaction
In patients with glaucoma (open-angle), a significant increase in the hypotensive effect of β-blockers (timolol and butylaminohydroxypropoxyphenoxymethyl methyloxadiazole) was noted when used together with Taufon.
The effect is enhanced by increasing the coefficient of ease of outflow and reducing the production of aqueous humor.
Overdose
Overdose
Data
there are no reports of overdose.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
4 years
Manufacturer
Manufacturer
Pharmstandard-UfaVITA, Russia
Additional information
| Shelf life | 4 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Pharmstandard-UfaVITA, Russia |
| Medication form | eye drops |
| Brand | Pharmstandard-UfaVITA |
Related products
Buy Taufon, eye drops 4% 10 ml with delivery to USA, UK, Europe and over 120 other countries.