No products in the cart.
Tamsulosin-Vertex, 0.4 mg capsules 90 pcs
€31.00 €25.83
Description
Tamsulosin is a specific blocker of postsynaptic α1-adrenoreceptors located in the smooth muscles of the prostate, bladder neck and prostatic part of the urethra. Blockade of α1-adrenoreceptors by tamsulosin leads to decrease of tone of the smooth muscles of the prostate, bladder neck and prostatic part of the urethra and improves outflow of urine.
The severity of symptoms of filling and emptying associated with increased smooth muscle tone and detrusor hyperactivity in benign prostatic hyperplasia are simultaneously reduced. < br>
The ability of tamsulosin to affect α1A subtype of adrenoreceptors is 20 times greater than its ability to interact with α1B subtype of adrenoreceptors located in vascular smooth muscle. < br>
Owing to its high selectivity, tamsulosin does not cause clinically significant reduction of systemic arterial pressure (BP) both in patients with arterial hypertension and in patients with normal baseline BP.
< br>.
Indications
Indications
Treatment of dysuric disorders in benign prostatic hyperplasia.
Pharmacological effect
Pharmacological effect
Tamsulosin is a specific blocker of postsynaptic α1-adrenergic receptors located in the smooth muscles of the prostate gland, bladder neck and prostatic urethra. Blockade of α1-adrenergic receptors by tamsulosin leads to a decrease in the tone of the smooth muscles of the prostate gland, bladder neck and prostatic urethra and improves urine outflow.
At the same time, the severity of symptoms of filling and emptying, caused by increased smooth muscle tone and detrusor hyperactivity in benign prostatic hyperplasia, decreases.
The ability of tamsulosin to act on the α1A subtype of adrenergic receptors is 20 times greater than its ability to interact with the α1B subtype of adrenergic receptors, which are located in vascular smooth muscle.
Due to its high selectivity, tamsulosin does not cause a clinically significant decrease in systemic blood pressure (BP) both in patients with arterial hypertension and in patients with normal baseline blood pressure.
Special instructions
Special instructions
At the first signs of orthostatic hypotension (dizziness, weakness), you should sit or lie down until these symptoms disappear.
Before starting treatment with tamsulosin, it is necessary to evaluate the patient to exclude other diseases that can cause the same symptoms as benign prostatic hyperplasia.
Before starting treatment with the drug and during therapy, you should regularly examine the prostate gland and, if necessary, determine prostate specific antigen (PSA). Due to the possibility of complications during cataract surgery, it is not recommended to prescribe tamsulosin treatment to patients with cataracts if surgery is planned.
When taking tamsulosin, drowsiness, blurred vision, dizziness and fainting are possible, so the drug should be used with caution while working by vehicle drivers and people whose profession requires increased concentration.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, it is necessary to refrain from engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Tamsulosin
Composition
Composition
Extended release capsules.
1 extended-release capsule contains:
Tamsulosin hydrochloride substance-pellets 0.2% – 200.0 mg [active substance: tamsulosin hydrochloride – 0.4 mg; excipients: sugar spheres (sucrose – 99%, hypromellose – 1%) – 190.0 mg, methacrylic acid copolymer – 4.8 mg, ethylcellulose – 4.0 mg, polyethylene glycol – 0.8 mg].
Hard gelatin capsules:
titanium dioxide – 1.0%,
iron oxide yellow (iron oxide) – 0.27%,
patented blue dye – 0.015%,
gelatin – up to 100%.
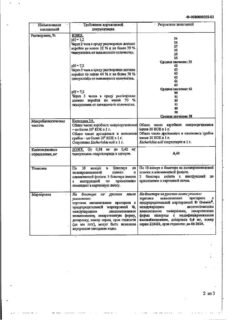
Contraindications
Contraindications
Hypersensitivity to tamsulosin hydrochloride or other components of the drug Tamsulosin;
Orthostatic hypotension (including history);
Severe liver failure.
With caution:
Severe renal failure (decrease in creatinine clearance below 10 ml/min).
Side Effects
Side Effects
From the nervous system: often – dizziness, infrequently – headache, rarely – fainting; sleep disturbance (drowsiness or insomnia);
From the cardiovascular system: infrequently – tachycardia, orthostatic hypotension; chest pain;
From the respiratory system: infrequently – rhinitis.
From the digestive system: infrequently – constipation, diarrhea, nausea, vomiting.
From the skin and skin: infrequently – rash, itching, urticaria; rarely – angioedema.
From the genitourinary system: infrequently – retrograde ejaculation, very rarely – priapism; decreased libido;
Others: sometimes – asthenia, back pain, small pupil syndrome during cataract surgery
Interaction
Interaction
No interaction was observed when tamsulosin was taken concomitantly with atenolol, enalapril, nifedipine or theophylline. When taken simultaneously with cimetidine, the concentration of tamsulosin in the blood plasma increases, and when taken simultaneously with tamsulosin with furosemide, it decreases.
However, no dose adjustment is required since tamsulosin concentrations remain within the therapeutic range.
In vitro, diazepam, propranolol, trichlormethiazide, chlormadinone, amitriptyline, diclofenac, glibenclamide, simvastatin and warfarin do not change the level of the free fraction of tamsulosin in the blood plasma. In turn, tamsulosin does not change the content of free fractions of diazepam, propranolol, trichlormethiazide and chlormadinone in the blood plasma.
There was no interaction of tamsulosin with amitriptyline, salbutamol, glibenclamide and finasteride in in vitro studies. When taken simultaneously with tamsulosin, diclofenac or warfarin may increase the rate of elimination of tamsulosin.
When taking tamsulosin simultaneously with drugs that lower blood pressure, including other alpha1-blockers and anesthetics, the hypotensive effect may be enhanced.
Overdose
Overdose
No cases of overdose have been described; acute hypotension is theoretically possible.
Treatment: in case of overdose, it is recommended to transfer the patient to a horizontal position and take measures aimed at maintaining the function of the cardiovascular system (restoring blood pressure and heart rate); gastric lavage, administration of activated carbon and osmotic laxatives such as sodium sulfate.
If there is no effect, substances that increase the volume of circulating blood and, if necessary, vasoconstrictors should be administered. Kidney function should be monitored and measures aimed at maintaining it should be taken. Dialysis is not very effective in cases of tamsulosin overdose, since the drug is largely bound to plasma proteins.
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Manufacturer | Vertex, Russia |
|---|---|
| Medication form | slow-release capsules |
| Brand | Vertex |
Other forms…
Related products
Buy Tamsulosin-Vertex, 0.4 mg capsules 90 pcs with delivery to USA, UK, Europe and over 120 other countries.