No products in the cart.
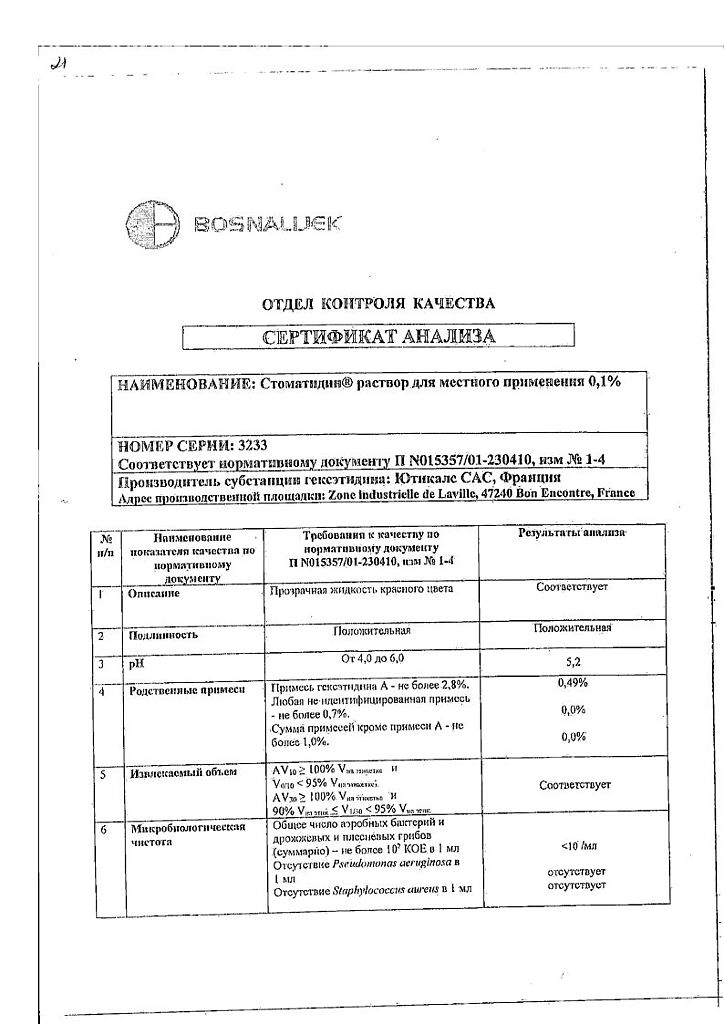
Stomatidine, 0.1% solution 200 ml
€10.38 €8.65
Description
Stomatidine is an antiseptic drug for topical use in otorhinolaryngology and dentistry.
The antimicrobial action of hexatidine is associated with the inhibition of oxidative reactions of metabolism of microbial cells (thiamine antagonist). The drug has antibacterial action against Gram-negative and Gram-positive bacteria, Pseudomonas aeruginosa and Proteus spp. and antifungal action (including against fungi of the genus Candida). At a concentration of 100 mg/ml inhibition of growth of most bacterial strains is achieved. No development of resistance was observed.
Hexethidine has a weak analgesic effect on the mucosa.
Pharmacokinetics
Hexethidine adheres well to the mucosa and is practically not absorbed. After a single application, hexatidine is detectable on the oral mucosa for 65 hours. In dental plaques active concentrations persist for 10-14 hours after application.
Indications
Indications
As a symptomatic remedy.
Symptomatic treatment for infectious and inflammatory diseases of the oral cavity and larynx:
– tonsillitis, tonsillitis (including Plaut-Vincent tonsillitis, tonsillitis of the lateral ridges), pharyngitis, gingivitis, stomatitis, glossitis, periodontal disease;
– fungal diseases;
– prevention of infectious complications before and after surgical interventions in the oral cavity and larynx, and in case of injuries, incl. prevention of infection of the alveoli after tooth extraction;
– oral hygiene, incl. to eliminate bad breath.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
Drugs used in dentistry. Antimicrobial agents and antiseptics for local treatment of oral diseases.
ATX code: А01АВ12
Pharmacological properties:
The antimicrobial effect of the drug is associated with the suppression of oxidative reactions in the metabolism of microbial cells (thiamine antagonist). The drug has a wide spectrum of antibacterial and antifungal effects, in particular against gram-positive bacteria and fungi of the genus Candida. Hexethidine has a weak anesthetic effect on the mucous membrane. The drug has an antiviral effect against influenza A viruses, respiratory syncytial virus (RS virus), herpes simplex virus type 1, which affects the respiratory tract.
Pharmacokinetics:
Hexethidine adheres well to the mucous membrane and is practically not absorbed. After a single use, hexethidine is detected on the oral mucosa for 65 hours. In dental plaques, active concentrations remain for 10-14 hours after application.
Special instructions
Special instructions
Do not use the drug in those categories of patients who cannot carry out the rinsing procedure on their own.
The drug Stomatidin® contains the dye azorubine CI 14720 (E122), which can cause allergic reactions (including delayed ones).
The drug contains propylene glycol, which may cause irritation to the skin and mucous membranes.
The drug Stomatidin® contains 0.219 mmol (or 0.5 mg) sodium in one tablespoon. In case of accidental ingestion, this should be taken into account in patients on a sodium-restricted diet.
The drug Stomatidin® contains 10 vol. % ethanol (alcohol), that is, up to 1.21 g in one tablespoon, which corresponds to 30 ml of beer or 15 ml of wine. If swallowed, harmful to persons with alcoholism, liver disease, epilepsy, trauma or brain disease, or pregnant women.
Impact on the ability to drive vehicles and machinery
The drug does not affect the ability to drive vehicles or operate machinery.
The drug contains 10% ethanol (ethyl alcohol 96%). The drug should be used no later than 30 minutes before driving.
Active ingredient
Active ingredient
Hexethidine
Composition
Composition
1 ml of solution contains:
Active ingredient – hexethidine 1.00 mg
Excipients: propylene glycol, polysorbate-20, citric acid monohydrate, sodium saccharinate, racementol, methyl salicylate, azorubine dye, ethanol 96%, water.
Pregnancy
Pregnancy
There is no information about any undesirable effects of hexetidine during pregnancy and breastfeeding. However, before prescribing Stomatidin® to pregnant or lactating women, the physician should carefully weigh the benefits and risks of treatment, given the lack of sufficient data on the penetration of the drug through the placenta and into breast milk.
Contraindications
Contraindications
Hypersensitivity to any of the components of the drug.
Erosive-desquamatous lesions of the oral mucosa.
Children under 3 years of age.
With caution:
Hypersensitivity to acetylsalicylic acid. Children’s age from 3 to 18 years. Liver disease, alcoholism, brain injury or disease, women during pregnancy.
Side Effects
Side Effects
The frequency of occurrence of adverse reactions is indicated in accordance with the rule: very often (≥1/10), often (≥1/100, but ˂1/10), infrequently (≥1/1000, but ˂1/100), rarely (≥1/10000, but ˂1/1000), very rarely (˂1/10,000), frequency unknown (based on It is impossible to estimate the available data).
Immune system disorders
Very rare: hypersensitivity reactions (including urticaria, angioedema).
Nervous system disorders
Very rare: ageusia, dysgeusia.
Disorders of the respiratory system, chest and mediastinal organs
Very rare: cough, shortness of breath due to a hypersensitivity reaction.
Gastrointestinal disorders
Very rare: dry mouth, dysphagia, nausea, enlarged salivary glands, vomiting.
General disorders and reactions at the injection site
Very rare: application site reactions (including irritation of the oral and pharyngeal mucosa, burning sensation, oral paresthesia, discoloration of the tongue, discoloration of the teeth, inflammation, blistering and ulceration).
If any of the side effects listed in the instructions get worse, or you notice other side effects, it is recommended to consult a doctor.
Interaction
Interaction
Not described.
Overdose
Overdose
It is unlikely that hexethidine can cause toxic effects when used according to the instructions for use of the medicinal product.
Symptoms: Ingestion of large quantities of hexethidine solution containing ethanol may result in signs/symptoms of alcohol intoxication.
In any case of overdose, consult your doctor immediately.
Treatment: symptomatic treatment, as with alcohol intoxication.
Gastric lavage is necessary within 2 hours after ingestion of an excess dose.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C. Do not freeze.
Keep out of the reach of children!
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Bosnalek JSC, Bosnia and Herzegovina
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | Bosnalek AS, Bosnia and Herzegovina |
| Medication form | topical solution |
| Brand | Bosnalek AS |
Related products
Buy Stomatidine, 0.1% solution 200 ml with delivery to USA, UK, Europe and over 120 other countries.