No products in the cart.
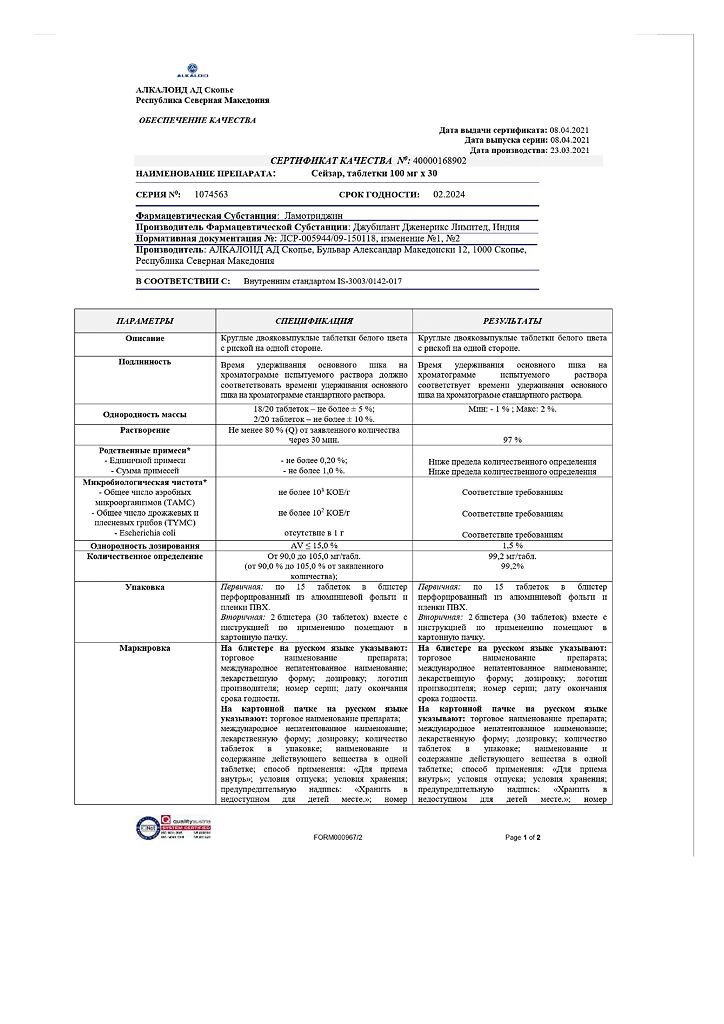
Seizar, tablets 100 mg 30 pcs
€32.59 €27.16
Description
Lamotrigine is a blocker of potential-dependent sodium channels. It reduces pathological activity of neurons without suppressing their function. It stabilizes neuronal membranes through its effect on Na+channels, blocks excessive glutamate release without reducing its normal release.
Pharmacokinetics
Absorption. Lamotrigine is rapidly and completely absorbed from the intestine, undergoing virtually no presystemic first-pass metabolism. Maximum plasma concentration is reached approximately 2.5 hours after oral administration. The time to reach maximum concentration is slightly longer after meals, but the degree of absorption remains unchanged. Pharmacokinetics is linear with a single dose of up to 450 mg (the highest dose studied). Significant interindividual variation in maximum equilibrium concentration is observed, however, with rare variations in each individual.
Distribution. Lamotrigine binds to plasma proteins by approximately 55%. It is unlikely that release of the drug from protein binding can lead to the development of toxic effects. The volume of distribution is 0.92-1.22 L/kg.
Metabolism. The enzyme uridine diphosphate-glucuronyltransferase (UDF-glucuronyltransferase) is involved in the metabolism of lamotrigine. Lamotrigine increases its own metabolism to a small extent in a dose-dependent manner. However, there is no evidence that lamotrigine affects the pharmacokinetics of other antiepileptic drugs and that interactions are possible between lamotrigine and other drugs metabolized by the cytochrome P450 system.
Excretion. In healthy adults, lamotrigine clearance at equilibrium concentrations averages 39±14 mL/min.
Lamotrigine is metabolized to glucuronides, which are excreted by the kidneys. Less than 10% of the drug is excreted unchanged by the kidneys, about 2% – by the intestine. Clearance and elimination half-life do not depend on the dose. Half-life in healthy adults is on average 24 hours to 35 hours. In patients with Gilbert’s syndrome a decrease in drug clearance by 32% was observed compared to the control group, which, however, was within normal values for the general population.
The half-life of lamotrigine is greatly influenced by the drugs taken together.
The average half-life is reduced to approximately 14 h when co-administered with glucuronizing drugs such as carbamazepine and phenytoin, and increased to an average of 70 h when co-administered with valproic acid.
Special patient groups
Children
In children lamotrigine clearance per body weight is higher than in adults; it is highest in children under 5 years of age. The elimination half-life of lamotrigine in children is usually shorter than in adults. It averages approximately 7 h when concomitantly administered with glucuronizing agents such as carbamazepine and phenytoin and increases to an average of 45-50 h when concomitantly administered with valproic acid.
Elderly patients
No clinically significant differences in lamotrigine clearance have been found in elderly patients compared to younger patients.
Patients with impaired renal function
If renal function is impaired, the initial dose of lamotrigine is calculated according to the standard antiepileptic drug regimen. A dose reduction may be required only if renal function is significantly impaired.
Patients with impaired hepatic function
The initial, increasing, and maintenance doses should be reduced by approximately 50% in patients with moderate hepatic impairment (Child-Pugh stage B) and by 75% in patients with severe hepatic impairment (Child-Pugh stage C). Dose increases and maintenance doses should be adjusted according to clinical effect.
Clinical efficacy in patients with bipolar disorder
Efficacy in preventing mood disorders in patients with bipolar disorder has been demonstrated in two basic clinical studies.
Combined analysis of the findings found that duration of remission, defined as time before first episode of depression and before first episode Duration of remission was more pronounced for depression.
Indications
Indications
Epilepsy
Adults and children (over 12 years old)
epilepsy (partial and generalized seizures, including tonic-clonic seizures, as well as seizures in Lennox-Gastaut syndrome) as part of combination therapy or monotherapy.
Children from 3 to 12 years old
epilepsy (partial and generalized seizures, including tonic-clonic seizures, as well as seizures in Lennox-Gastaut syndrome) as part of combination therapy. Once control of epilepsy is achieved with combination therapy, concomitant antiepileptic drugs (AEDs) can be discontinued and lamotrigine continued in monotherapy.
Monotherapy for typical absence seizures.
Bipolar disorders
Adults (18 years and older)
to prevent mood disorders (depression, mania, hypomania, mixed episodes) in patients with bipolar disorder.
Pharmacological effect
Pharmacological effect
Lamotrigine is a voltage-gated sodium channel blocker. Reduces the pathological activity of neurons without inhibiting their function. Stabilizes neuronal membranes by influencing Na+ channels, blocks excessive release of glutamate without reducing its normal release.
Pharmacokinetics
Suction. Lamotrigine is rapidly and completely absorbed from the intestine, with virtually no first-pass metabolism. Maximum plasma concentrations are achieved approximately 2.5 hours after oral administration of the drug. The time to reach maximum concentration increases slightly after eating, but the extent of absorption remains unchanged. Pharmacokinetics is linear when taking a single dose of up to 450 mg (the highest dose studied). There is significant interindividual variation in the maximum concentration at steady state, however, with rare variations within each individual.
Distribution. Lamotrigine is approximately 55% bound to plasma proteins. It is unlikely that the release of the drug from its protein binding could lead to the development of a toxic effect. The volume of distribution is 0.92-1.22 l/kg.
Metabolism. The enzyme uridine diphosphate-glucuronyltransferase (UDP-glucuronyltransferase) is involved in the metabolism of lamotrigine. Lamotrigine slightly increases its own metabolism in a dose-dependent manner. However, there is no evidence that lamotrigine affects the pharmacokinetics of other antiepileptic drugs and that interactions are possible between lamotrigine and other drugs metabolized by the cytochrome P450 system.
Excretion. In healthy adults, the clearance of lamotrigine at steady state concentrations averages 39±14 ml/min.
Lamotrigine is metabolized to glucuronides, which are excreted by the kidneys. Less than 10% of the drug is excreted unchanged by the kidneys, about 2% by the intestines. Clearance and half-life are independent of dose. The half-life in healthy adults averages from 24 hours to 35 hours. In patients with Gilbert’s syndrome, a decrease in drug clearance by 32% was observed compared with the control group, which, however, did not exceed the normal values for the general population.
The half-life of lamotrigine is greatly influenced by concomitantly administered drugs.
The mean elimination half-life decreases to approximately 14 hours when coadministered with drugs that stimulate glucuronidation, such as carbamazepine and phenytoin, and increases to an average of 70 hours when coadministered with valproic acid.
Special groups of patients
Children
In children, the clearance of lamotrigine based on body weight is higher than in adults; it is highest in children under 5 years of age. The half-life of lamotrigine is generally shorter in children than in adults. Its average value is approximately 7 hours when co-administered with drugs that stimulate glucuronidation, such as carbamazepine and phenytoin, and increases to an average of 45-50 hours when co-administered with valproic acid.
Elderly patients
No clinically significant differences in the clearance of lamotrigine were found in elderly patients compared with young patients.
Patients with impaired renal function
If renal function is impaired, the initial dose of lamotrigine is calculated in accordance with the standard antiepileptic drug regimen. A dose reduction may only be necessary if there is a significant decrease in renal function.
Patients with liver dysfunction
Initial, escalating and maintenance doses should be reduced by approximately 50% in patients with moderate hepatic impairment (Child-Pugh stage B) and by 75% in patients with severe hepatic impairment (Child-Pugh stage C). Dose escalation and maintenance dosage should be adjusted based on clinical response.
Clinical effectiveness in patients with bipolar disorders
Effectiveness in preventing mood disorders in patients with bipolar disorder has been demonstrated in two seminal clinical studies.
As a result of a combined analysis of the results obtained, it was found that the duration of remission, defined as the time until the onset of the first episode of depression and until the first episode of mania/hypomania/mixed after stabilization, was longer in the lamotrigine group compared with placebo. The duration of remission is more pronounced for depression.
Special instructions
Special instructions
Use with caution in patients with renal failure.
Lamotrigine should not be used in elderly patients.
If severe allergic skin reactions occur, use of lamotrigine should be discontinued.
If lamotrigine is suddenly discontinued, the manifestations of epilepsy may increase, so it is necessary to gradually stop treatment, reducing the dose over 2 weeks.
When used simultaneously with carbamazepine, dizziness, diplopia, ataxia, visual impairment, and nausea are possible. These phenomena usually disappear when the dose of carbamazepine is reduced.
Lamotrigine should not be used in children under 2 years of age.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, a slowdown in the speed of psychomotor reactions is observed. This must be taken into account by persons engaged in potentially hazardous activities that require increased attention and rapid psychomotor reactions.
Active ingredient
Active ingredient
Lamotrigine
Composition
Composition
Active substance: one tablet contains lamotrigine 100 mg.
Excipients: low-substituted hyprolose, calcium carbonate, sodium carboxymethyl starch, colloidal silicon dioxide, magnesium aluminosilicate, magnesium stearate, sodium saccharinate, povidone, microcrystalline cellulose, blackcurrant flavor.
Pregnancy
Pregnancy
Clinical data on the safety of lamotrigine during pregnancy and lactation are insufficient.
When deciding whether to use it during pregnancy, the expected benefits of therapy for the mother should be weighed against the potential risk to the fetus.
Preliminary data indicate that lamotrigine is excreted in breast milk at concentrations that are 40-45% of plasma concentrations.
A small number of infants whose mothers received lamotrigine did not experience any side effects.
Use in children
Lamotrigine should not be used in children under 2 years of age.
Contraindications
Contraindications
Hypersensitivity to lamotrigine or any component of the drug. Children up to 3 years of age (for this dosage form).
With caution
History of chronic renal failure, allergic reactions or skin rash to other antiepileptic drugs.
Side Effects
Side Effects
From the side of the central nervous system: headache, dizziness, drowsiness, sleep disturbances, feeling tired, aggressiveness, confusion.
From the digestive system: nausea, liver dysfunction.
From the hematopoietic system: leukopenia, thrombocytopenia.
Allergic reactions: skin rash (usually maculopapular), angioedema, Stevens-Johnson syndrome, toxic epidermal necrolysis, lymphadenopathy.
Interaction
Interaction
When used simultaneously with anticonvulsants – inducers of metabolism in the liver (including phenytoin, carbamazepine, phenobarbital, primidone), the metabolism of lamotrigine is accelerated.
With the simultaneous use of lamotrigine and carbamazepine or phenytoin, the T1/2 of lamotrigine decreases. There have been reports of dizziness, ataxia, diplopia, blurred vision and nausea in patients taking carbamazepine after starting treatment with lamotrigine.
Due to the inhibition of microsomal liver enzymes under the influence of sodium valproate, with simultaneous use the metabolism of lamotrigine slows down and T1/2 of lamotrigine increases.
Overdose
Overdose
Single administration doses exceeding the maximum therapeutic dose by 10–20 times have been reported.
Symptoms: nystagmus, ataxia, disturbances of consciousness to the point of coma.
Treatment: hospitalization and appropriate symptomatic therapy. In case of recent (less than 2 hours) use of the drug, gastric lavage is necessary.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 15–25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Alkaloid AD Skopje, Republic of North Macedonia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place at 15-25 °C |
| Manufacturer | Alkaloid AD Skopje, Republic of Northern Macedonia |
| Medication form | pills |
| Brand | Alkaloid AD Skopje |
Other forms…
Related products
Buy Seizar, tablets 100 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.