Subtotal: €40.39
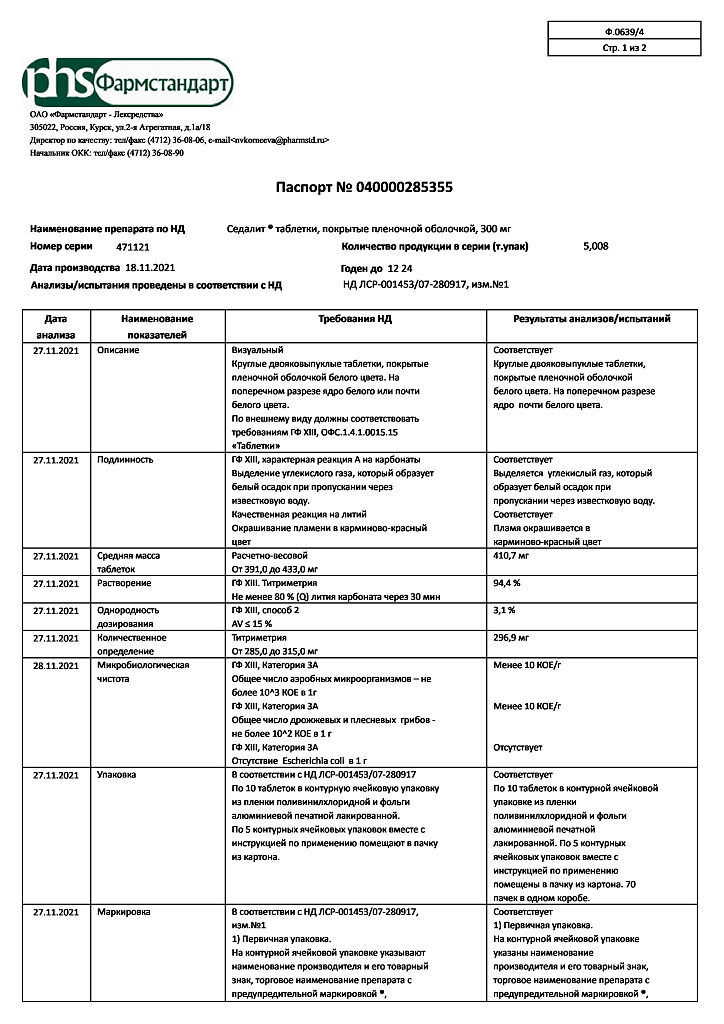
Sedalit, 300 mg 50 pcs.
€10.66 €8.89
Out of stock
(E-mail when Stock is available)
Sedalit is a normothetic drug (normalizes the mental state without causing general lethargy).
Sedalite also has antidepressant, sedative and antimanic effects. The effect is due to the lithium ions, which, being antagonists of sodium ions, displace them from the cells and thus reduce the bioelectrical activity of brain neurons.
Accelerates the breakdown of biogenic amines (decreases the concentration of norepinephrine and serotonin in brain tissue).
Sedalite increases the sensitivity of neurons of the hippocampus and other areas of the brain to the action of dopamine. It interacts with lipids formed during inositol metabolism.
Pharmacokinetics
When taken orally it is absorbed from the gastrointestinal tract. Cmax of the active substance in plasma is reached after approximately 9 hours.
It does not bind with plasma proteins.
It penetrates the placental barrier, is excreted with breast milk. It is not metabolized.
It is excreted by the kidneys – 95%, with the feces – less than 1%, with perspiration – 4-5%.
Indications
Manic and hypomanic states of various origins,
prevention and treatment of affective psychoses,
prevention and treatment of affective disorders in patients with chronic alcoholism.
Migraine, Meniere’s syndrome, sexual disorders, drug addiction.
Pharmacological effect
Sedalite is a normothimic (normalizes the mental state without causing general lethargy).
Sedalite also has antidepressant, sedative and antimanic effects. The effect is caused by lithium ions, which, being antagonists of sodium ions, displace them from cells and thereby reduce the bioelectrical activity of brain neurons.
Accelerates the breakdown of biogenic amines (the concentration of norepinephrine and serotonin in brain tissue decreases).
Sedalite increases the sensitivity of neurons in the hippocampus and other areas of the brain to the effects of dopamine. Interacts with lipids formed during the metabolism of inositol.
Pharmacokinetics
When taken orally, it is absorbed from the gastrointestinal tract. Cmax of the active substance in plasma is reached after approximately 9 hours.
Does not bind to plasma proteins.
Penetrates the placental barrier and is excreted in breast milk. Not metabolized.
Excreted by the kidneys – 95%, with feces – less than 1%, through sweating – 4-5%.
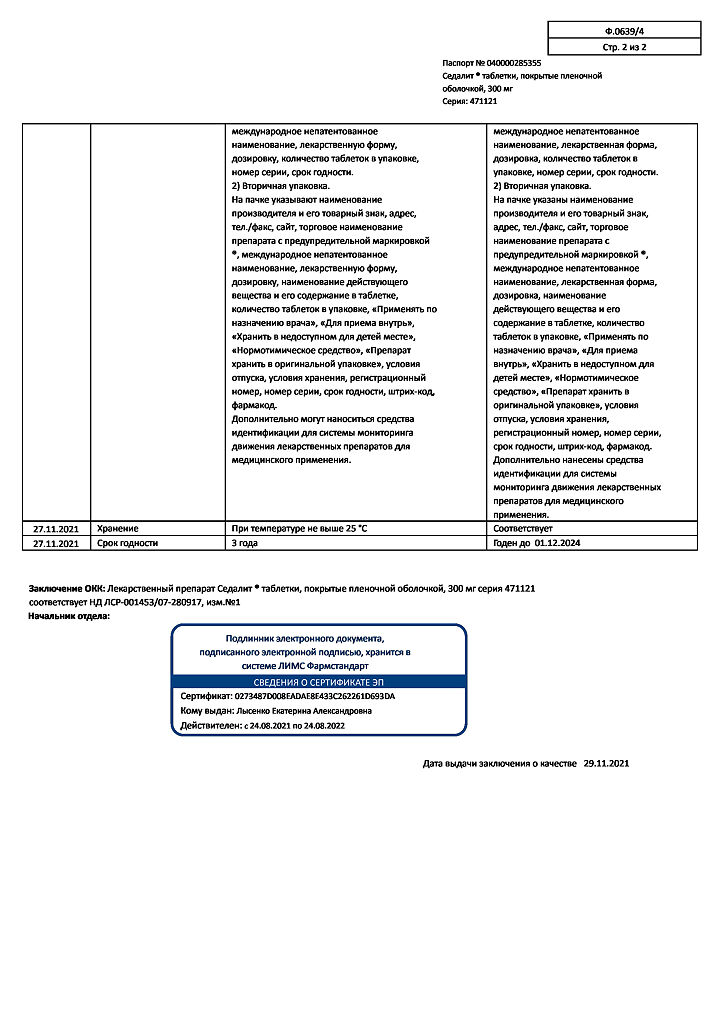
Special instructions
Use with caution in cardiovascular diseases (including AV block, intraventricular block), central nervous system diseases (including epilepsy, parkinsonism, organic lesions, schizophrenia), severe dehydration, infectious diseases, urinary retention, renal failure, as well as diabetes, hyperparathyroidism, thyrotoxicosis, psoriasis, in debilitated patients and with hyponatremia of any etiology.
Elderly and debilitated patients require dosage adjustment.
Nausea and vomiting, early signs of lithium toxicity, may be masked by the antiemetic effect of some phenothiazines.
During the first month of therapy, the concentration of lithium in the blood plasma should be monitored weekly. When a stable concentration is achieved, monitoring is carried out monthly, then once every 2-3 months.
During the treatment period, avoid drinking alcohol.
Long-acting dosage forms should not be used in children under 12 years of age and should not be alternated with other dosage forms.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care should be taken when driving vehicles and engaging in other potentially hazardous activities that require increased attention and speed of psychomotor reactions.
Active ingredient
Lithium carbonate
Composition
1 film-coated tablet contains:
active substance:
lithium carbonate 300 mg
Contraindications
Hypersensitivity to lithium and other components of the drug; severe surgical interventions; leukemia; pregnancy, breastfeeding period; children under 12 years of age (efficacy and safety of use have not been established); diseases of the cardiovascular system associated with heart rhythm disturbances; presence of Brugada syndrome/family history of Brugada syndrome; severe renal failure; untreated or non-compensable hypothyroidism; low levels of sodium in the body, for example in patients with dehydration, patients on a salt-free diet or patients with Addison’s disease.
With caution
Diseases of the cardiovascular system (including atrioventricular and intraventricular block), diseases of the central nervous system (epilepsy, parkinsonism), diabetes mellitus, thyrotoxicosis, hyperparathyroidism, infections, psoriasis, renal failure, urinary retention.
Side Effects
From the central nervous system and peripheral nervous system: muscle weakness, hand tremors, adynamia, drowsiness; with prolonged use, articulation disorders and hyperreflexia are possible.
From the cardiovascular system: heart rhythm disturbances.
From the digestive system: dyspepsia.
From the endocrine system: rarely – dysfunction of the thyroid gland.
Other: increased thirst, impaired hematopoiesis, leukocytosis, increased body weight.
Interaction
When used simultaneously with thiazide diuretics and indapamide, a rapid increase in the concentration of lithium in the blood plasma and the development of toxic effects are possible.
When used simultaneously with ACE inhibitors, it is possible to increase the concentration of lithium in the blood plasma and develop toxic effects; with NSAIDs – the toxic effects of lithium may be enhanced; with iodine preparations – there may be an increased risk of thyroid dysfunction; with xanthine derivatives – it is possible to increase the excretion of lithium in the urine, which can lead to a decrease in its effectiveness.
When used simultaneously with alprazolam, a clinically significant increase in the concentration of lithium in the blood plasma is possible; with acyclovir – a case of increased toxic effects of lithium has been described; with baclofen – cases of increased hyperkinetic symptoms in patients with Huntington’s chorea have been described.
When lithium carbonate is used simultaneously with verapamil, drug interactions are unpredictable. With the simultaneous use of lithium carbonate with diltiazem, a case of the development of psychosis has been described.
When used simultaneously with haloperidol, extrapyramidal symptoms may increase; with carbamazepine, clonazepam – the development of neurotoxicity is possible.
When used simultaneously with methyldopa, lithium toxicity may develop; with metronidazole – it is possible to increase the concentration of lithium in the blood plasma.
When used simultaneously with sodium chloride, sodium bicarbonate, high sodium intake increases the excretion of lithium, which may lead to a decrease in its effectiveness.
When used simultaneously with norepinephrine, the vasoconstrictor effect of norepinephrine may be reduced; with phenytoin – cases of the development of symptoms of lithium toxicity have been described; with fluoxetine – it is possible to increase the concentration of lithium in the blood plasma and develop toxic effects; with furosemide and bumetanide, cases of increased toxic effects of lithium have been described.
When used simultaneously with chlorpromazine and other phenothiazines, it is possible to reduce the absorption of phenothiazines from the gastrointestinal tract and reduce their concentration in the blood plasma by 40%, increase the intracellular concentration of lithium and the rate of its excretion in the urine, increase the risk of developing extrapyramidal reactions, delirium, and cerebellar dysfunction (especially in the elderly).
Storage conditions
In a dry place, protected from light
Shelf life
3 years
Manufacturer
Pharmstandard-Leksredstva, Russia
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | pills |
| Brand | Pharmstandard-Leksredstva |
Related products
Buy Sedalit, 300 mg 50 pcs. with delivery to USA, UK, Europe and over 120 other countries.