No products in the cart.
Rhinofluimucil, spray 10 ml
€10.47 €8.72
Description
Pharmacotherapeutic group:antagonist.
ATX code: R01AB08.
Pharmacological Properties
Pharmacodynamics
. The mucolytic and vasoconstrictor effect of Rinofluimucil® is a reflection of the pharmacological properties of its active ingredients.
Acetylcysteine has mucolytic activity due to free sulphhydryl group breaking disulphide bonds of mucus glycoproteins and having thinner effect on nasopharyngeal secretion.
Tuaminoheptane sulphate is a sympathomimetic amine having vasoconstrictor effect without systemic action when applied topically.
The two substances act synergistically to reduce intranasal resistance.
Pharmacokinetics
Absorption
After administration of the therapeutic dose (2 pressings of 50 µL) of Rinofluimucil®, the maximum plasma concentration of tuaminohepatan is reached between 0.25 h and 6.0 h.
Distribution
The mean Cmax value of tuaminoheptane after 2 50 µl pressings of Rinofluimucil® was 0.95 ng/ml, the mean Tmax value was 2 h.
Biotransformation
The metabolism of tuaminoheptane sulfate in human hepatocytes has been studied in vitro. No transformation occurred in human hepatocytes under in vitro conditions. The major metabolite of N-acetylcysteine is inorganic sulfate, which is excreted in the urine; other metabolites include taurine, cysteine, and N,N-diacetylcysteine. N-acetylcysteine is rapidly deacetylated to cysteine, which is incorporated into proteins. Excess cysteine enters the liver, where it is either metabolized for excretion or further modified.
Elimation
The average plasma half-life of tuaminoheptane after 2 50 µl pressings of Rinofluimucil® is 9.8 hours. Twelve hours after administration, tuaminoheptane was detected in all subjects (mean 0.42 ng/ml). Twenty-four hours after administration, the mean plasma concentration of tuaminoheptane was 0.30 ng/ml, but in most subjects the concentration was already below the limit of quantification (0.100 ng/ml).
Indications
Indications
Acute and subacute rhinitis with thick purulent mucous secretion, chronic rhinitis, vasomotor rhinitis, atrophic rhinitis, sinusitis.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: anticongestive agent.
ATX code: R01AB08.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamics
The mucolytic and vasoconstrictor effect of Rinofluimucil® is a reflection of the pharmacological properties of the active substances included in its composition.
Acetylcysteine has mucolytic activity due to the presence of a free sulfhydryl group, which, by breaking the disulfide bonds of mucus glycoproteins, has a diluting effect on nasopharyngeal secretions.
Tuaminoheptane sulfate is a sympathomimetic amine; when applied topically, it has a vasoconstrictor effect without systemic effects.
These two substances act synergistically to reduce intranasal resistance.
Pharmacokinetics
Absorption
After administration of a therapeutic dose (2 injections of 50 μl) of Rinofluimucil®, the maximum concentration of tuaminohepatane in plasma is achieved in the range of 0.25 hours – 6.0 hours.
Distribution
The average Cmax value of tuaminoheptane after 2 injections of 50 μl of Rinofluimucil® was 0.95 ng/ml, the average Tmax value was 2 hours.
Biotransformation
The metabolism of tuaminoheptane sulfate in human hepatocytes has been studied in vitro. Under in vitro conditions, no transformation occurred in human hepatocytes. The main metabolite of N-acetylcysteine is inorganic sulfate, which is excreted in the urine; other metabolites include taurine, cysteine and N,N-diacetylcysteine. N-acetylcysteine is quickly deacetylated to cysteine, which is incorporated into proteins. Excess cysteine goes to the liver, where it is either metabolized for elimination or further modified.
Removal
The average half-life of tuaminoheptane in the blood plasma after 2 injections of 50 μl of Rinofluimucil® is 9.8 hours. 12 hours after administration, tuaminoheptane was determined in all subjects (average value 0.42 ng/ml). 24 hours after administration, the average concentration of tuaminoheptane in the blood plasma was 0.30 ng/ml, but in most subjects the concentration was already below the limit of quantitation (0.100 ng/ml).
Special instructions
Special instructions
In patients with cardiovascular diseases, especially patients with arterial hypertension, treatment should be carried out under the supervision of a physician.
The drug should be used with caution in patients with asthma. Rinofluimucil® should be used with caution in children, taking into account that the drug is contraindicated for use in children under 6 years of age.
Long-term use of drugs that constrict blood vessels can interfere with the normal function of the mucous membrane of the nasal cavity and paranasal sinuses, and can also cause addiction to the drug. Therefore, frequent use over a long period of time may have adverse effects.
The drug should be used with caution in elderly patients with prostatic hypertrophy due to the risk of urinary retention.
Use, especially long-term, of topical agents may
cause sensitization: in this case, it is necessary to stop using the drug and, if necessary, resort to appropriate treatment. If there is no complete therapeutic response within several days, you should consult your doctor; in any case, the duration of treatment should not exceed one week.
According to the doctor’s decision, the use of the drug can be combined with appropriate antibacterial therapy.
Tuaminoheptane sulfate may give a positive result in a doping test.
This drug is not intended for ophthalmic use.
INFLUENCE ON THE ABILITY TO DRIVE VEHICLES, MECHANISMS
Does not affect the ability to drive vehicles and machinery.
No specific studies have been conducted, but patients should be informed that hallucinations have been reported in some cases.
Active ingredient
Active ingredient
Acetylcysteine, Tuaminoheptane
Composition
Composition
100 ml of solution contains
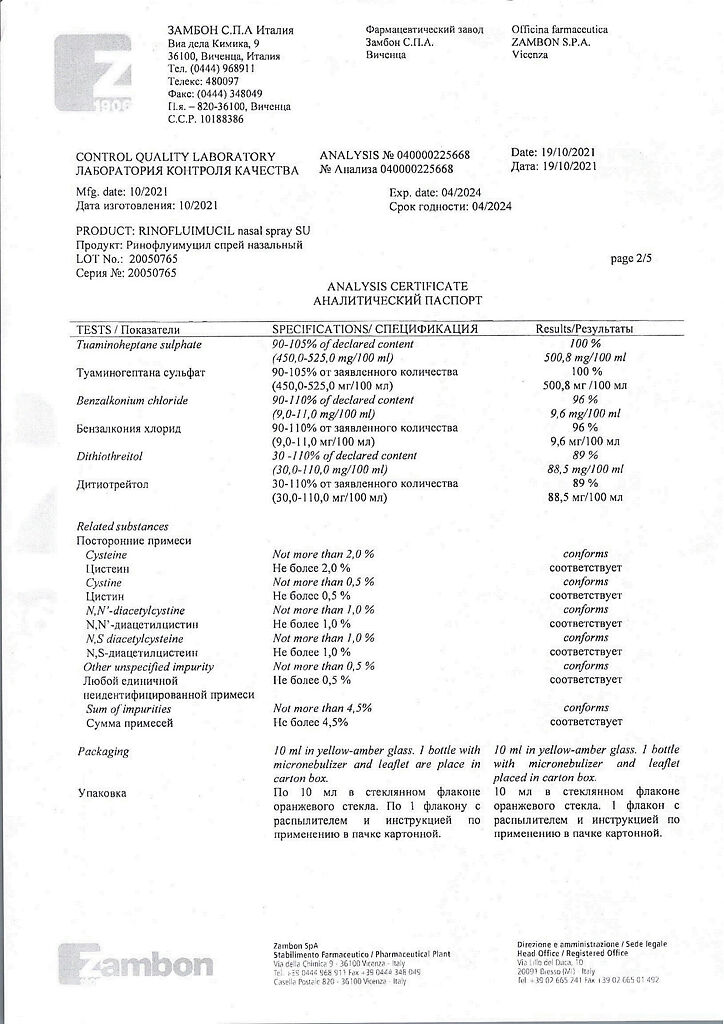
active ingredients: acetylcysteine – 1.0 g, tuaminoheptane sulfate – 0.5 g;
excipients: benzalkonium chloride 0.01 g, hypromellose 0.75 g, disodium edetate 0.02 g, sodium dihydrogen phosphate 0.3 g, sodium hydrogen phosphate dodecahydrate 0.3 g, dithiothreitol 0.1 g, sorbitol 70% 2.0 g, mint flavor 0.0188 g, ethanol 96% 0.31 g, sodium hydroxide 0.36 g, purified water up to 100 ml.
Pregnancy
Pregnancy
Data from a limited number of patients taking this drug during pregnancy do not indicate an adverse effect of acetylcysteine on the course of pregnancy or on the health of the fetus/newborn child. To date, no other relevant epidemiological data are available. Animal studies of acetylcysteine do not indicate direct or indirect harmful effects in terms of reproductive toxicity.
There are no data on the use during pregnancy or on animal studies of tuaminoheptane or the combination of acetylcysteine with tuaminoheptane.
The use of this medicine during pregnancy is not recommended.
Although there is no information on the excretion of acetylcysteine or tuaminoheptane into breast milk, a risk to breastfed infants cannot be excluded. The use of this medicine during breastfeeding is not recommended.
Contraindications
Contraindications
– Hypersensitivity to the active substance or any of the excipients.
– Cardiovascular diseases, including hypertension.
– History of cerebrovascular disease, including the presence of relevant risk factors (due to alpha-sympathomimetic activity).
– History of seizures.
– Pheochromocytoma.
– Angle-closure glaucoma.
– Concomitant use of other sympathomimetic nasal agents, including other nasal decongestants.
– Patients who are currently receiving or have received within 2 weeks monoamine oxidase inhibitors, including reversible inhibitors of monoamine oxidase A (RIMA).
– Hypophysectomy or surgery to expose the dura mater.
– Children under 6 years of age.
With caution:
– Occlusive vascular diseases.
– Diabetes mellitus.
– Hyperthyroidism.
– Asthma.
– Prostate hypertrophy, as it can make urination difficult.
– Use of beta blockers.
– Long-term use may cause resumption of congestion symptoms and drug-induced rhinitis.
Side Effects
Side Effects
Frequent use of the drug in high dosages can cause side effects of a sympathomimetic nature (such as increased excitability, rapid heartbeat, tremor, etc.). Sometimes dry nose and throat, as well as acne, are possible. These reactions, however, completely disappear when you stop taking the drug.
The adverse reactions listed below may be associated with taking Rinofluimucil®; the incidence of these adverse reactions is unknown (cannot be estimated based on available data):
System-organ class
Adverse reaction
Immune disorders
systems
Hypersensitivity
Mental disorders
Especially with prolonged and/or frequent use:
anxiety, hallucinations, delusions
Nervous system disorders
Especially with prolonged and/or frequent use: headache, anxiety, agitation,
insomnia, tremor
Heart disorders
Especially with prolonged and/or
frequent use: frequent
palpitations, tachycardia, arrhythmia
Vascular disorders
Hypertension
Disorders of the chest and mediastinum
Especially with prolonged and/or frequent use: dry nose and throat, feeling
nasal discomfort, stuffiness
nose
Gastrointestinal disorders
intestinal tract:
Nausea
Skin disorders and
subcutaneous tissues
Urticarial rash, rash
Kidney disorders and
urinary tract
Urinary retention
General disorders and reactions at the injection site
Especially with prolonged and/or
frequent use: irritation at the injection site, addiction
Interaction
Interaction
Despite the low systemic absorption of tuaminoheptane, when it is applied topically to the nasal cavity, the following potential interactions should be taken into account:
– Monoamine oxidase inhibitors (MAOIs), including reversible monoamine oxidase inhibitors (rMAOIs): increased risk of hypertensive crisis;
– Antihypertensive drugs (including adrenergic neuron blockers or beta blockers): may block the hypotensive effect of drugs;
– Cardiac glycosides: may increase the risk of cardiac arrhythmia;
– Ergot alkaloids: may increase the risk of ergotism;
– Drugs for the treatment of Parkinson’s disease: may increase the risk of cardiovascular toxicity;
– Oxytocin: may increase the risk of hypertension;
Overdose
Overdose
In case of overdose, adult patients may experience the following symptoms: arterial hypertension, photophobia, severe headache, chest tightness.
In case of overdose in children, hypothermia with pronounced sedation is possible.
Treatment: symptomatic.
Storage conditions
Storage conditions
At temperatures from 15 to 25 °C.
Keep out of the reach of children
Shelf life
Shelf life
2.5 years.
After opening the bottle, the contents can be used within 20 days.
Do not use after expiration date.
Manufacturer
Manufacturer
Zambon S.p.A., Italy
Additional information
| Shelf life | 2.5 years. After opening the bottle, the contents can be used within 20 days. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At 15 to 25 ° C. Keep out of reach of children |
| Manufacturer | Zambon S.p.A., Italy |
| Medication form | nasal spray |
| Brand | Zambon S.p.A. |
Related products
Buy Rhinofluimucil, spray 10 ml with delivery to USA, UK, Europe and over 120 other countries.