No products in the cart.
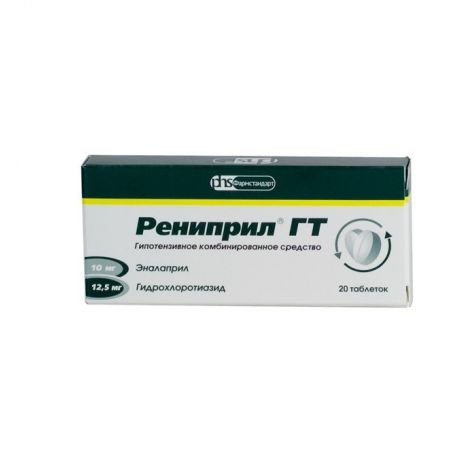
Renipril GT, tablets 12.5mg+10 mg 20 pcs
€5.07 €4.51
Description
Pharmacotherapeutic group
Hypertensive combination medicine (angiotensin-converting enzyme inhibitor (ACE)+ diuretic)
ATX code: [C09BA02]
Pharmacological properties
Pharmacodynamics
Renipril® GT is a combination drug containing the active ingredients: enalapril and hydrochlorothiazide, whose action is due to the properties of its constituent components.
Enalapril is an angiotensin-converting enzyme (ACE) inhibitor and is a “prodrug”: its hydrolysis creates enalapril, which inhibits ACE.
Hydrochlorothiazide is a thiazide diuretic. It acts at the level of the distal renal tubules, increasing the excretion of sodium and chlorine ions. At the beginning of treatment with hydrochlorothiazide, the volume of fluid in the vessels decreases as a result of increased sodium and fluid excretion, which leads to a decrease in blood pressure (BP) and a decrease in cardiac output. With continued therapy, the hypotensive effect of hydrochlorothiazide is based on a decrease in total peripheral vascular resistance. Enalapril enhances antihypertensive effect – inhibits renin-angiotensin-aldosterone system, i.e. angiotensin II production and its effects. Additionally, it reduces the production of aldosterone and increases the effects of bradykinin and the release of prostaglandins. Since it often has its own diuretic effect, this may increase the effect of hydrochlorothiazide.
Enalapril reduces pre- and postload, which unloads the left ventricle, leading to regression of hypertrophy. As a result, the heart rhythm slows down and the load on the heart (in chronic heart failure) decreases, coronary blood flow improves and oxygen consumption by cardiomyocytes decreases. Thus, the sensitivity of the heart to ischemia is reduced. It has a favorable effect on the cerebral blood flow in patients with arterial hypertension and chronic cardiovascular disease. It prevents the development of glomerulosclerosis, maintains and improves kidney function and slows the course of chronic kidney disease even in patients who have not yet developed arterial hypertension.
The antihypertensive effect of ACE inhibitors is greater in patients with hyponatremia, hypovolemia and elevated serum renin levels, whereas the effect of hydrochlorothiazide is independent of serum renin levels. Therefore, simultaneous administration of enalapril and hydrochlorothiazide has an additional antihypertensive effect. In addition, enalapril prevents or attenuates the metabolic effects of diuretic therapy and has a favorable effect on structural changes in the heart and vessels.
The simultaneous prescription of ACE inhibitor and hydrochlorothiazide is used when each drug separately is not sufficiently effective or monotherapy is performed using maximum doses of the drug, which increases the incidence of adverse effects. The antihypertensive effect of the combination usually lasts for 24 hours.
Pharmacokinetics
Enalapril is rapidly absorbed from the gastrointestinal tract. The volume of absorption is 60%. Food has no effect on the absorption of enalapril. Maximum plasma concentration is reached after 1 hour. Enalapril is hydrolyzed in the liver to the active metabolite enalaprilate. Maximum concentration of enalapril in blood serum is reached after 3-6 hours. Enalapril is excreted with urine (60%) and feces (33%) mainly as enalaprilate. Enalaprilat penetrates into most body tissues, mainly lungs, kidneys and blood vessels. Blood plasma protein binding is 50-60 %. Renal clearance of enalapril and enalaprilat is 0.005 ml/s (18 l/h) and 0.00225-0.00264 ml/s (8.1-9.5 l/h) respectively. It is excreted in several steps. With multiple doses of enalapril, the serum elimination half-life of enalaprilat is approximately 11 hours. Enalapril and enalaprilat penetrate the placental barrier and are excreted with breast milk.
Enalaprilat is eliminated from the bloodstream by hemodialysis or peritoneal dialysis. Hemodialysis clearance of enalaprilat is 0.63-1.03 mL/s (38-62 mL/min); serum concentration of enalaprilat decreases by 45-57% after 4 hours of hemodialysis.
In patients with reduced renal function, excretion is slower, requiring dosage adjustments according to renal function, particularly in patients with significant renal impairment.
In patients with hepatic impairment, the metabolism of enalapril may be delayed with no change in its pharmacodynamic effect.
In patients with heart failure the absorption and metabolism of enalaprilat is delayed and the volume of distribution is also reduced.
Hydrochlorothiazide is absorbed primarily in the duodenum and proximal small intestine. Absorption is 70% and increases by 10% when ingested with food. The level of maximum serum concentration is reached after 1.5-5 hours. The volume of distribution is about 3 l/kg. Binding to plasma proteins is 40%. The drug accumulates in erythrocytes, the mechanism of accumulation is not known. It is not metabolized in liver, it is excreted mainly by kidneys: 95% unchanged and about 4% as hydrolysate of 2-amino-4-chloro-m-benzenedisulfonamide. Renal clearance of hydrochlorothiazide in healthy volunteers and patients with arterial hypertension is approximately 5.58 ml/s (335 ml/min). Hydrochlorothiazide has a biphasic excretion profile. The elimination half-life (T1/2) in the initial phase is 2 h, in the final phase (10-12 h after administration) – about 10 h. It penetrates through the placental barrier and accumulates in the amniotic fluid. The serum concentration of hydrochlorothiazide in umbilical vein blood is almost the same as in maternal blood. The concentration in amniotic fluid is 19 times higher than in serum from the umbilical vein. The level of hydrochlorothiazide in breast milk is very low.
When hydrochlorothiazide is administered to patients with heart failure, it has been found that its absorption is reduced in proportion to the degree of the disease – by 20-70%. T1/2 hydrochlorothiazide increases to 28.9 hours; renal clearance is 0.17-3.12 ml/s (10-187 ml/min) (mean values 1.28 ml/s (77 ml/min)).
In patients who have undergone intestinal bypass surgery for obesity, absorption of hydrochlorothiazide may be reduced by 30% and serum concentrations by 50% than in healthy volunteers.
The concomitant administration of enalapril and hydrochlorothiazide has no effect on the pharmacokinetics of either.
Indications
Indications
Arterial hypertension (including renovascular), congestive heart failure.
Active ingredient
Active ingredient
Composition
Composition
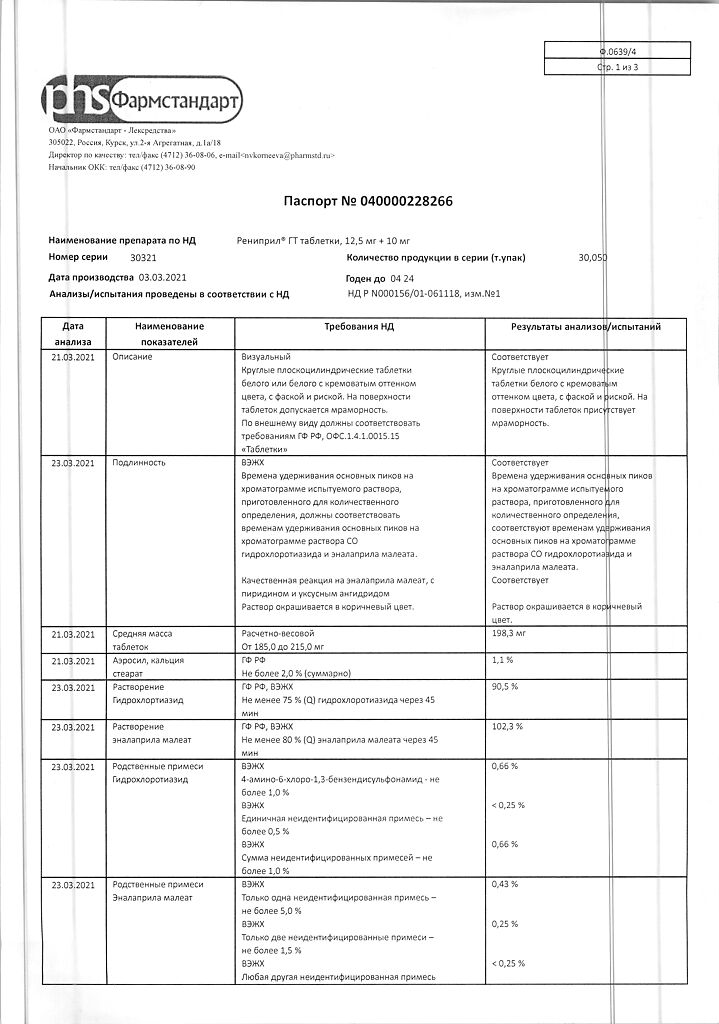
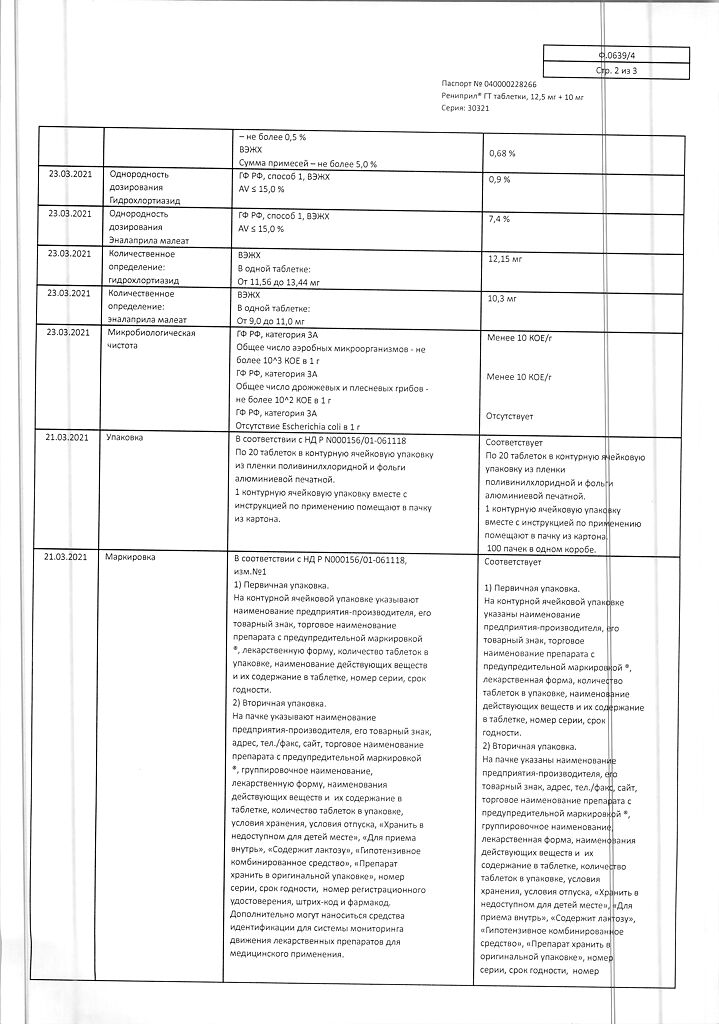
Composition per tablet:
The active ingredients:
enalapril maleate – 10 mg,
hydrochlorothiazide – 12.5 mg.
Auxiliary substances:
potato starch – 59.3 mg,
lactose monohydrate (milk sugar) – 111.5 mg,
povidone (polyvinylpyrrolidone low molecular weight medical) – 4 mg,
colloidal silicon dioxide (aerosil) – 0.7 mg,
calcium stearate (calcium stearate) – 2 mg.
How to take, the dosage
How to take, the dosage
The treatment of arterial hypertension does not begin with a combination of medications. Initially, adequate doses of individual components must be determined. The dosage must always be chosen individually for each patient.
The usual dose is 1 tablet per day. Patients should take the tablets whole during or after meals, with a small amount of liquid, preferably in the morning. If the next dose of the drug is missed, it should be taken as soon as possible, if there is a long enough time before the next dose. If it is a few hours before the next dose, you should wait and take only this dose. You should never double the dose.
If satisfactory therapeutic effect is not achieved, it is recommended that another medication be added or therapy changed.
In patients on diuretic therapy, it is recommended to discontinue treatment or reduce the dose of diuretics at least 3 days before treatment with Renipril® GT to prevent the development of symptomatic hypotension. Renal function should be investigated before starting treatment.
Dosage in impaired renal function
Patients with creatinine clearance greater than 0.5 mL/s or serum creatinine less than 265 mol/L (3 mg/100 mL) may be prescribed the usual dose of Renipril® GT.
Interaction
Interaction
It enhances the effect of other hypotensive drugs, barbiturates. NSAIDs weaken the antihypertensive effect, potassium-saving diuretics, potassium preparations increase the risk of hyperkalemia.
Delays excretion of lithium, increases toxicity of cardiac glycosides. Hydrochlorothiazide weakens the effect of antidiabetic drugs, renipril increases it.
Contraindications
Contraindications
– hypersensitivity (including to individual drug components or sulfonamides)
– anuria, severe renal impairment (creatinine clearance less than 30 ml/min)
– bilateral stenosis of the renal arteries or stenosis of the arteries of the single kidney
– angioedema in anamnesis, connected with the use of ACE inhibitors, as well as hereditary or idiopathic angioedema
– primary hyperaldosteronism
– Addison’s disease
– porphyria
– pregnancy, lactation
– age under 18 years (effectiveness and safety are unknown).
With caution
– renal function disorders (creatinine clearance 30-75 ml/min)
– marked aortic orifice stenosis or idiopathic hypertrophic subaortic stenosis
– Ischemic heart disease and cerebrovascular disease (including insufficient cerebral circulation)
– Chronic heart failure
– Severe autoimmune systemic connective tissue diseases (including systemic lupus erythematosus, scleroderma) – bone marrow suppression
– diabetes
– hyperkalemia
– conditions after kidney transplantation
– severe liver function disorders
– conditions accompanied by a decrease in circulating blood volume (as a result of diuretic therapy, salt restriction, diarrhea, and vomiting)
– advanced age
– gout.
Side effects
Side effects
Nervous system and sensory organs: dizziness, headache, weakness, increased fatigue, decreased libido.
Cardiovascular system and blood (hematopoiesis, hemostasis): hypotension, collapse, neutropenia, hyperkalemia.
Gastrointestinal system disorders: nausea, liver function disorders (increased liver transaminases activity, bilirubin level), diarrhea.
Others: cough, skin rash, renal dysfunction, proteinuria, muscle cramps, angioedema.
Pregnancy use
Pregnancy use
It is contraindicated in pregnancy.
Breastfeeding should be stopped during treatment.
Similarities
Similarities
Additional information
| Weight | 0.011 kg |
|---|---|
| Shelf life | 3 years |
| Conditions of storage | In a dry, light-protected place |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | pills |
| Brand | Pharmstandard-Leksredstva |
Related products
Buy Renipril GT, tablets 12.5mg+10 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.