No products in the cart.
Relifipine, gel 20 mg/g+3 mg/g 20 g
€26.72 €22.27
Description
Pharmacotherapeutic group
Hemorrhoid treatment
ATX code: C05AXO3.
Pharmacological properties Pharmacodynamics
Nifedipine
Nifedipine is a selective slow calcium channel blocker, a 1,4-dihydropyridine derivative. The mechanism of action in rectal administration is associated with reduction of extracellular calcium flow inside the smooth muscle cells of the sphincter of the anus. Nifedipine eliminates hypertonicity of the sphincter of the anus, which in turn leads to a reduction in the severity of pain in patients with hemorrhoids and anal fissures. Decrease of sphincter sphincter tone of the anal canal improves blood supply to ischemic zones of the mucous membrane of the anal canal and improves its functional condition. Pharmacological activity of nifedipine causes reduction of the intensity of the inflammatory reaction and has a modulating effect on the microcirculation.
Lidocaine
Lidocaine is a local anesthetic. Its mechanism of action when used rectally is due to disruption of the functional activity of sodium channels, which prevents the generation of pain impulses in the endings of sensitive nerves and conduct of pain impulses along the nerve fibres. The analgesic effect develops 1-5 minutes after application of the drug to the skin or mucous membranes and lasts 30-60 minutes.
Pharmacokinetics
Nifedipine is practically not absorbed when applied topically, being determined in blood plasma at therapeutically insignificant concentration, insufficient for determination of other pharmacokinetic parameters.
Lidocaine when applied to the mucous membranes is absorbed and enters the systemic bloodstream. It is metabolized in the liver (90-95%) by oxidative N-dealkylation, hydrolysis of the amide bond and hydroxylation of the aromatic ring to form 4-hydroxy-2,6-xylidine, which is the main metabolite. It is excreted mainly by the kidneys as this metabolite.
Indications
Indications
Treatment of anal fissures due to chronic hemorrhoids; symptomatic treatment of acute hemorrhoids.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Hemorrhoids treatment remedy
ATX code: С05АХО3.
Pharmacological properties Pharmacodynamics
Nifedipine
Nifedipine is a selective blocker of slow calcium channels, a 1,4-dihydropyridine derivative. The mechanism of action when administered rectally is associated with a decrease in the flow of extracellular calcium into the smooth muscle cells of the anal sphincter. Nifedipine eliminates hypertonicity of the anal sphincter, which, in turn, leads to a decrease in the severity of pain in patients with hemorrhoids and anal fissure. Reducing the tone of the anal sphincter helps improve blood supply to ischemic areas of the mucous membrane of the anal canal and improve its functional state. The pharmacological activity of nifedipine causes a decrease in the intensity of the inflammatory reaction and has a modulating effect on microcirculation.
Lidocaine
Lidocaine is a local anesthetic. The mechanism of action when used rectally is due to a violation of the functional activity of sodium channels, which prevents the generation of pain impulses in the endings of sensory nerves and the conduction of pain impulses along nerve fibers. The analgesic effect develops 1–5 minutes after applying the drug to the skin or mucous membranes and lasts 30–60 minutes.
Pharmacokinetics
Nifedipine, when used externally, is practically not absorbed, being determined in the blood plasma in a therapeutically insignificant concentration, insufficient to determine other pharmacokinetic parameters.
Lidocaine, when applied to the mucous membranes, is absorbed and enters the systemic circulation. Metabolized in the liver (90–95%) by oxidative Ndealkylation, hydrolysis of the amide bond and hydroxylation of the aromatic ring to form 4-hydroxy-2,6-xylidine, which is the main metabolite. It is excreted primarily by the kidneys in the form of this metabolite.
Special instructions
Special instructions
It is necessary to consult with your doctor regarding the absence of indications for surgical treatment of anal fissure.
Ingestion of the gel or contact with eyes should be avoided. If the gel accidentally gets into your eyes, rinse them with sufficient water.
After using the gel, you must wash your hands thoroughly.
In a clinical study, the effectiveness and safety of treatment with Relifipin was assessed in patients with anal fissure associated with stage 1–2 chronic hemorrhoids. Efficacy has not been assessed in patients with anal fissure secondary to stage 3–4 chronic hemorrhoids and in the presence of more than one anal fissure. If you have a history of rectal surgery, Relifipin should be used with caution and under the supervision of a physician.
Impact on the ability to drive vehicles and machinery
Not identified.
Active ingredient
Active ingredient
Lidocaine, Nifedipine
Composition
Composition
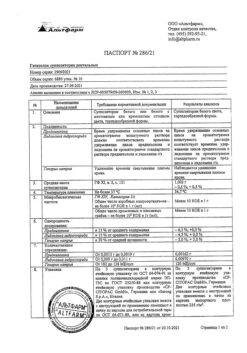
1 g of the drug contains:
Active ingredients:
Lidocaine hydrochloride monohydrate 0.0213 g (in terms of lidocaine hydrochloride) (0.0200 g)
Nifedipine 0.0030 g
Excipients:
Poloxamer 188 0.0500 g
Poloxamer 407 0.1750 g
Polyethylene oxide 400 (Macrogol 400) 0.3000 g
Methyl parahydroxybenzoate (nipagin) 0.0007 g
Sodium hydroxide (sodium hydroxide) 0.0007 g
Propyl parahydroxybenzoate (nipazole) 0.0003 g
Purified water up to 1 g
Pregnancy
Pregnancy
The combination drug lidocaine + nifedipine has not been studied in pregnant women and during breastfeeding. Contraindicated for use during pregnancy and breastfeeding.
Contraindications
Contraindications
Hypersensitivity to the components of the drug; age under 18 years; pregnancy; breastfeeding period; anal fissure, complicated by the presence of an abscess, fistula; oncological diseases of the large intestine and rectum; rectal stenosis; severe arterial hypotension, chronic heart failure with unstable hemodynamic parameters, Crohn’s disease, signs of infection of the rectum.
With caution
History of surgical interventions on the rectum.
Side Effects
Side Effects
Allergic reactions, anaphylactic shock, very rarely – reactions at the injection site (pain, burning, itching, hyperemia), rectal bleeding.
Interaction
Interaction
The interaction of the combination drug lidocaine + nifedipine with other drugs has not been studied in experimental studies in animals. With simultaneous use of the drug with antiarrhythmic drugs, the QT interval may be prolonged, and in very rare cases, ventricular fibrillation may develop. When using the drug simultaneously with nitrate-containing drugs, in very rare cases, arterial hypotension may develop.
Overdose
Overdose
In case of accidental ingestion of the drug (swallowing of several grams of gel) or rectal administration of an excess amount of the drug, the most severe reactions are possible from the cardiovascular system (decreased blood pressure, bradycardia, bradyarrhythmia, depression of sinus node function, cardiac arrest) and the central nervous system (convulsions, respiratory depression, respiratory arrest). Treatment is symptomatic.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C. Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
St. Petersburg pharmaceutical factory, Russia
Additional information
| Shelf life | 2 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | At a temperature not higher than 25 ° C. Keep out of reach of children. |
| Manufacturer | St. Petersburg Pharmaceutical Factory, Russia |
| Medication form | gel for rectal and external use |
| Brand | St. Petersburg Pharmaceutical Factory |
Other forms…
Related products
Buy Relifipine, gel 20 mg/g+3 mg/g 20 g with delivery to USA, UK, Europe and over 120 other countries.