No products in the cart.
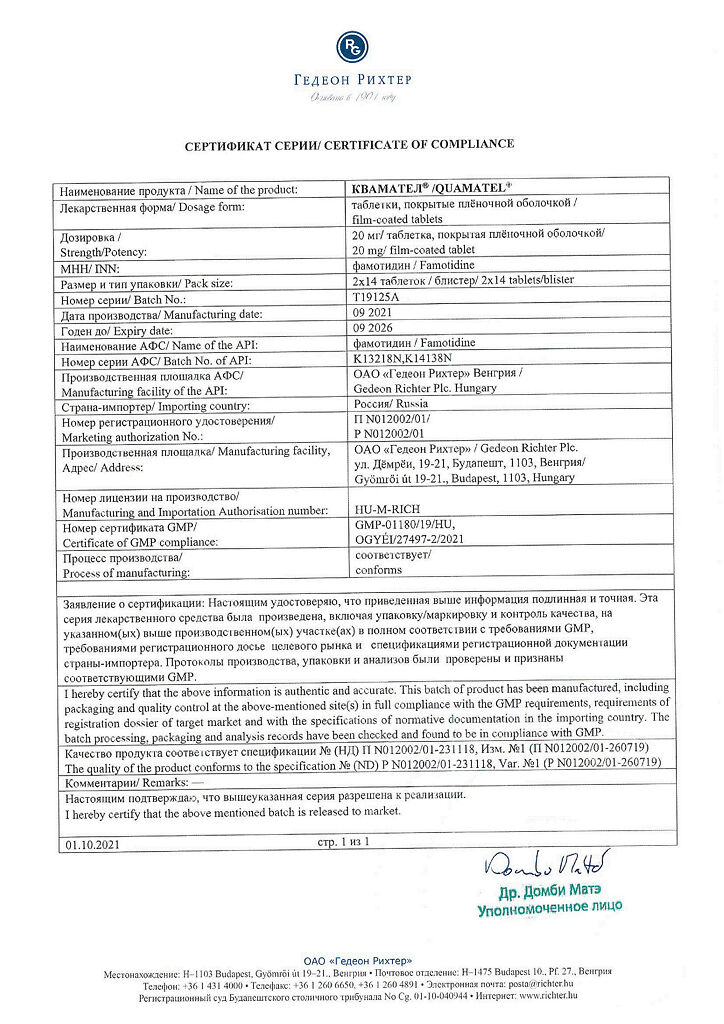
Quamatel, 20 mg 28 pcs.
€5.01 €4.45
Description
Quamatel is a blocker of histamine H2-receptors.
It reduces basal and stimulated by gastrin, pentagastrin, betazol, caffeine, histamine, acetylcholine and physiological vagus reflex secretion of hydrochloric acid.
The pH increases and pepsin activity decreases. It has practically no effect on gastrin levels on an empty stomach and after meals. It does not affect gastric motility, exocrine activity of the pancreas, blood circulation in the portal system, hormone levels, and has no antiandrogenic effect.
Famotidine has little effect on microsomal liver enzymes.
After oral administration the action comes within 1 h, the maximum action is within 3 h after administration, the duration of effect varies within 12-24 h, depending on the dose.
The drug in the form of lyophilisate for preparation of solution for intravenous injection after a single dose of 20 or 40 mg in the evening suppresses basal and nocturnal secretion for 10-12 hours.
Indications
Indications
peptic ulcer of the duodenum and stomach in the acute phase, prevention of relapses;
treatment and prevention of symptomatic ulcers of the stomach and duodenum (associated with taking NSAIDs, stress, post-operative);
erosive gastroduodenitis;
functional dyspepsia associated with increased secretory function of the stomach;
reflux esophagitis;
Zollinger-Ellison syndrome;
prevention of recurrent bleeding from the upper gastrointestinal tract;
prevention of aspiration of gastric juice during general anesthesia (Mendelssohn syndrome).
Pharmacological effect
Pharmacological effect
Kvamatel is a histamine H2 receptor blocker.
Reduces basal and stimulated by gastrin, pentagastrin, betazol, caffeine, histamine, acetylcholine and the physiological vagal reflex secretion of hydrochloric acid.
At the same time, pH increases and pepsin activity decreases. Has virtually no effect on fasting and postprandial gastrin levels. Does not affect gastric motility, exocrine activity of the pancreas, blood circulation in the portal system, hormone levels, and does not have an antiandrogenic effect.
Famotidine has little effect on liver microsomal enzymes.
After taking the drug orally, the effect occurs within 1 hour, the maximum effect occurs 3 hours after administration, the duration of the effect ranges from 12-24 hours, depending on the dose.
The drug in the form of a lyophilisate for the preparation of a solution for intravenous administration after administration in a single dose of 20 or 40 mg in the evening suppresses basal and nocturnal secretion for 10-12 hours.
Special instructions
Special instructions
The use of Kvamatel can mask the symptoms of stomach cancer, therefore, before starting treatment with famotidine, it is necessary to exclude the presence of a malignant neoplasm.
If treatment is abruptly stopped, famotidine may cause withdrawal syndrome, so treatment is stopped, gradually reducing its dose.
In patients with impaired liver function, Kvamatel should be prescribed with caution and in lower doses.
With long-term treatment of weakened patients or patients under stress, bacterial damage to the stomach is possible with further spread of infection.
Kvamatel should be taken 2 hours after taking itraconazole or ketoconazole to avoid a significant decrease in their absorption. You should also observe a 1-2 hour break between taking Kvamatel and antacids.
Histamine H2 receptor blockers (including Kvamatel) can inhibit the acid-stimulating effect of pentagastrin and histamine, so Kvamatel should be discontinued 24 hours before the test.
Histamine H2 receptor blockers may suppress the skin response to histamine, thus leading to false negative results. Therefore, before performing diagnostic skin tests to detect an immediate allergic skin reaction, Kvamatel should be discontinued.
During treatment, you should avoid consuming foods, drinks and other medications that may irritate the gastric mucosa.
Impact on the ability to drive vehicles and operate machinery
During the period of taking the drug Kvamatel, patients should be careful when driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Famotidine
Composition
Composition
1 tablet contains:
Active substance:
Contraindications
Contraindications
pregnancy;
lactation period (breastfeeding);
childhood;
hypersensitivity to the components of the drug;
hypersensitivity to other histamine H2 receptor blockers.
With caution: the drug should be prescribed for renal and liver failure, for cirrhosis of the liver with a history of portosystemic encephalopathy.
Side Effects
Side Effects
From the digestive system: dry mouth, nausea, vomiting, abdominal pain, flatulence, constipation, diarrhea, loss of appetite; increased activity of liver transaminases, hepatocellular, cholestatic or mixed hepatitis, acute pancreatitis.
From the hematopoietic system: very rarely – agranulocytosis, pancytopenia, leukopenia, thrombocytopenia, bone marrow hypo- or aplasia.
Allergic reactions: urticaria, skin rash, itching, bronchospasm, angioedema, anaphylactic shock.
From the cardiovascular system: arrhythmia, bradycardia, AV block, decreased blood pressure.
From the central nervous system: headache, dizziness, drowsiness, hallucinations, confusion, fatigue, depression, agitation, anxiety.
From the senses: decreased visual acuity, tinnitus.
From the reproductive system: with long-term use in high doses – hyperprolactinemia, gynecomastia, amenorrhea, decreased libido.
From the musculoskeletal system: arthralgia, muscle spasms.
Dermatological reactions: alopecia, acne vulgaris, dry skin, toxic epidermal necrolysis.
Other: fever.
Interaction
Interaction
Due to the increase in the pH of the gastric contents, Kvamatel, when used simultaneously, reduces the absorption of ketoconazole and itraconazole; increases the absorption of amoxicillin and clavulanic acid.
Antacids and sucralfate, used simultaneously with Quamatel, slow down the absorption of famotidine.
When taking Kvamatel and drugs that inhibit bone marrow hematopoiesis simultaneously, the risk of developing neutropenia increases.
Overdose
Overdose
Symptoms: vomiting, motor agitation, tremor, decreased blood pressure, tachycardia, collapse.
Treatment: gastric lavage, symptomatic and supportive therapy; hemodialysis.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 30 °C
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Gedeon Richter, Hungary
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 30 °C |
| Manufacturer | Gedeon Richter, Hungary |
| Medication form | pills |
| Brand | Gedeon Richter |
Other forms…
Related products
Buy Quamatel, 20 mg 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.