No products in the cart.
Pentalgin, 12 pcs.
€4.32 €3.84
Description
Pharmacotherapeutic group: analgesic (analgesic non-narcotic + non-steroidal anti-inflammatory drug + psychostimulant + antispasmodic + H1-histamine receptor blocker).
ATC code: N02BE71
Pharmacological properties
Combined drug, has analgesic, anti-inflammatory, antispasmodic, antipyretic effect.
Paracetamol is a non-narcotic analgesic which has antipyretic and analgesic effect caused by blockade of cyclooxygenase in the central nervous system and by influence on the centers of pain and thermoregulation.
Naproxen is non-steroidal anti-inflammatory drug which has anti-inflammatory, analgesic and antipyretic effect due to non-selective inhibition of cyclooxygenase activity which regulates synthesis of prostaglandins.
Caffeine causes dilatation of blood vessels in skeletal muscles, heart, kidneys; it increases mental and physical performance, eliminates fatigue and sleepiness; it increases permeability of histohematic barriers and increases bioavailability of non-narcotic analgesics, thus enhancing therapeutic effect. It has a tonic effect on cerebral vessels.
Drotaverine has myotropic antispasmodic effect caused by inhibition of phosphodiesterase IV and acts on smooth muscles in the gastrointestinal tract, biliary tract, genitourinary and vascular systems.
Pheniramine is a blocker of H1-histamine receptors. It has antispasmodic and mild sedative effects, reduces the phenomena of exudation, and enhances the analgesic effect of paracetamol and naproxen.
Indications
Indications
Pain syndrome of various origins, including pain in the joints, muscles, radiculitis, menstrual pain, neuralgia, dental pain and headaches (including headaches caused by cerebral vasospasm).
Pain syndrome associated with spasm of smooth muscles, including chronic cholecystitis, cholelithiasis, postcholecystectomy syndrome, renal colic.
Post-traumatic and postoperative pain syndrome, including those accompanied by inflammation.
Colds accompanied by fever (as symptomatic therapy).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: analgesic (non-narcotic analgesic + non-steroidal anti-inflammatory drug + psychostimulant + antispasmodic + H1-histamine receptor blocker).
ATX code: N02BE71
Pharmacological properties
The combined drug has analgesic, anti-inflammatory, antispasmodic, antipyretic effects.
Paracetamol is a non-narcotic analgesic, has an antipyretic and analgesic effect due to the blockade of cyclooxygenase in the central nervous system and its effect on the centers of pain and thermoregulation.
Naproxen is a non-steroidal anti-inflammatory drug that has anti-inflammatory, analgesic and antipyretic effects associated with non-selective suppression of the activity of cyclooxygenase, which regulates the synthesis of prostaglandins.
Caffeine – causes dilation of blood vessels in skeletal muscles, heart, kidneys; increases mental and physical performance, helps eliminate fatigue and drowsiness; increases the permeability of histohematic barriers and increases the bioavailability of non-narcotic analgesics, thereby enhancing the therapeutic effect. It has a tonic effect on the blood vessels of the brain.
Drotaverine – has a myotropic antispasmodic effect due to inhibition of phosphodiesterase IV, acts on smooth muscles in the gastrointestinal tract, biliary tract, genitourinary and vascular systems.
Pheniramine is a blocker of H1-histamine receptors. It has an antispasmodic and mild sedative effect, reduces exudation, and also enhances the analgesic effect of paracetamol and naproxen.
Special instructions
Special instructions
Avoid simultaneous use of the drug with other products containing paracetamol and/or other non-steroidal anti-inflammatory drugs, as well as with drugs to relieve the symptoms of colds, flu and nasal congestion.
When using the drug for more than 5–7 days, peripheral blood counts and the functional state of the liver should be monitored.
Paracetamol distorts the results of laboratory tests of glucose and uric acid in blood plasma.
If it is necessary to determine 17-ketosteroids, the drug should be discontinued 48 hours before the study. Please note that naproxen increases bleeding time.
The effect of caffeine on the central nervous system depends on the type of nervous system and can be manifested by both excitation and inhibition of higher nervous activity.
During the treatment period you should not drink alcoholic beverages.
Impact on the ability to drive vehicles and machinery
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reaction.
Active ingredient
Active ingredient
Paracetamol, Naproxen, Caffeine, Drotaverine, Pheniramine
Composition
Composition
Active ingredients:
paracetamol – 325.0 mg,
naproxen – 100.0 mg,
caffeine anhydrous – 50.0 mg,
drotaverine hydrochloride – 40.0 mg,
pheniramine maleate – 10.0 mg.
Excipients: microcrystalline cellulose – 128.0000 mg, potato starch – 55.3800 mg, croscarmellose sodium – 32.0000 mg, hyprolose (hydroxypropylcellulose) – 32.5200 mg, citric acid monohydrate – 3.0000 mg, butylated hydroxytoluene (H 321) – 0.3000 mg, magnesium stearate – 7.2000 mg, talc – 16.1200 mg, quinoline yellow dye (E 104) – 0.4608 mg, indigo carmine (E 132) – 0.0192 mg
Shell: hypromellose (hydroxypropyl methylcellulose) – 12.1700 mg, povidone (medium molecular weight polyvinylpyrrolidone, povidone-K25) – 3.8700 mg, polysorbate-80 (Tween-80) – 1.1000 mg, titanium dioxide – 3.4300 mg, talc – 4.2180 mg, quinoline yellow dye (E 104) – 0.2000 mg, indigo carmine (E 132) – 0.0127 mg,
or
Shell OPALRAY 13A210001 Green (OPADRY 13A210001 GREEN) – 25.0007 mg [hypromellose – 12.1700 mg, povidone – 3.8700 mg, polysorbate-80 – 1.1000 mg, titanium dioxide – 3.4300 mg, talc – 4.2180 mg, quinoline yellow – 0.2000 mg. FD&C Blue #2/ Indigo Carmine – 0.0127 mg].
Pregnancy
Pregnancy
The drug is contraindicated for use during pregnancy. If it is necessary to use the drug during lactation, breastfeeding should be stopped.
Contraindications
Contraindications
Hypersensitivity to the components of the drug, erosive and ulcerative lesions of the gastrointestinal tract (in the acute phase), gastrointestinal bleeding, complete or incomplete combination of bronchial asthma, recurrent nasal polyposis and paranasal sinuses and intolerance to acetylsalicylic acid or other non-steroidal anti-inflammatory drugs, including a history of severe liver and/or renal failure, depression bone marrow hematopoiesis, condition after coronary artery bypass grafting; severe organic diseases of the cardiovascular system (including acute myocardial infarction), paroxysmal tachycardia, frequent ventricular extrasystole, severe arterial hypertension, hyperkalemia, children under 18 years of age, pregnancy and lactation.
With caution: cerebrovascular diseases, diabetes mellitus, peripheral arterial diseases, ulcerative lesions of the gastrointestinal tract in the anamnesis, renal and liver failure of mild or moderate severity, viral hepatitis, alcoholic liver damage, benign hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndrome), epilepsy and a tendency to seizures, deficiency of glucose-6-phosphate dehydrogenase, old age.
If you have one of the listed diseases/conditions, be sure to consult your doctor before taking the drug.
Side Effects
Side Effects
Allergic reactions: skin rash, itching, urticaria, angioedema;
From the hematopoietic organs: thrombocytopenia, leukopenia, agranulocytosis, anemia, methemoglobinemia;
From the nervous system: agitation, anxiety, increased reflexes, tremor, headache, sleep disturbances, dizziness, decreased concentration;
From the cardiovascular system: palpitations, arrhythmias, increased blood pressure;
From the digestive system: erosive and ulcerative lesions of the gastrointestinal tract, nausea, vomiting, epigastric discomfort, abdominal pain, constipation, impaired liver function;
From the urinary system: impaired renal function;
From the senses: hearing loss, tinnitus, increased intraocular pressure in patients with angle-closure glaucoma;
Other: dermatitis, tachypnea (increased breathing).
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Interaction
When taking the drug Pentalgin simultaneously with barbiturates, tricyclic antidepressants, rifampicin, ethanol, the risk of hepatotoxicity increases (these combinations should be avoided).
Paracetamol enhances the effect of indirect anticoagulants and reduces the effectiveness of uricosuric drugs.
Long-term use of barbiturates reduces the effectiveness of paracetamol.
When used simultaneously with paracetamol and ethanol, the risk of acute pancreatitis increases.
Inhibitors of microsomal oxidation (including cimetidine) reduce the risk of hepatotoxic action of paracetamol.
Diflunisal increases the plasma concentration of paracetamol by 50%, which increases the risk of hepatotoxicity.
Naproxen can cause a decrease in the diuretic effect of furosemide, an increase in the effect of indirect anticoagulants, increases the toxicity of sulfonamides and methotrexate, reduces the excretion of lithium and increases its concentration in the blood plasma.
With the combined use of caffeine and barbiturates, primidone, anticonvulsants (hydantoin derivatives, especially phenytoin), it is possible to increase metabolism and increase the clearance of caffeine; while taking caffeine and cimetidine, oral contraceptives, disulfiram, ciprofloxacin, norfloxacin – a decrease in the metabolism of caffeine in the liver (slowing its excretion and increasing its concentration in the blood).
Concomitant use of caffeinated beverages and other CNS stimulants may result in excessive CNS stimulation.
When used concomitantly, drotaverine may weaken the antiparkinsonian effect of levodopa.
With simultaneous use of pheniramine with tranquilizers, hypnotics, MAO inhibitors, ethanol, the depressant effect on the central nervous system may be enhanced.
Overdose
Overdose
Symptoms: pale skin, anorexia (lack of appetite), abdominal pain, nausea, vomiting, gastrointestinal bleeding, agitation, restlessness, confusion, tachycardia, arrhythmia, hyperthermia (increased body temperature), frequent urination, headache, tremors or muscle twitching; epileptic seizures, increased activity of “liver” transaminases, hepatonecrosis, increased prothrombin time.
Symptoms of liver dysfunction may appear 12-48 hours after an overdose. In case of severe overdose, liver failure develops with progressive encephalopathy, coma, death; acute renal failure with tubular necrosis; arrhythmia, pancreatitis. If you suspect an overdose, you should immediately seek medical help.
Treatment: gastric lavage followed by taking activated charcoal. The specific antidote for paracetamol poisoning is acetylcysteine. The administration of acetylcysteine is relevant for 8 hours.
In case of gastrointestinal bleeding, it is necessary to administer antacids and gastric lavage with an ice-cold 0.9% sodium chloride solution; maintaining pulmonary ventilation and oxygenation; for epileptic seizures – intravenous administration of diazepam; maintaining fluid and salt balance.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 oC.
Keep out of the reach of children.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
OTCPharm Pro/Pharmstandard, Russia
Additional information
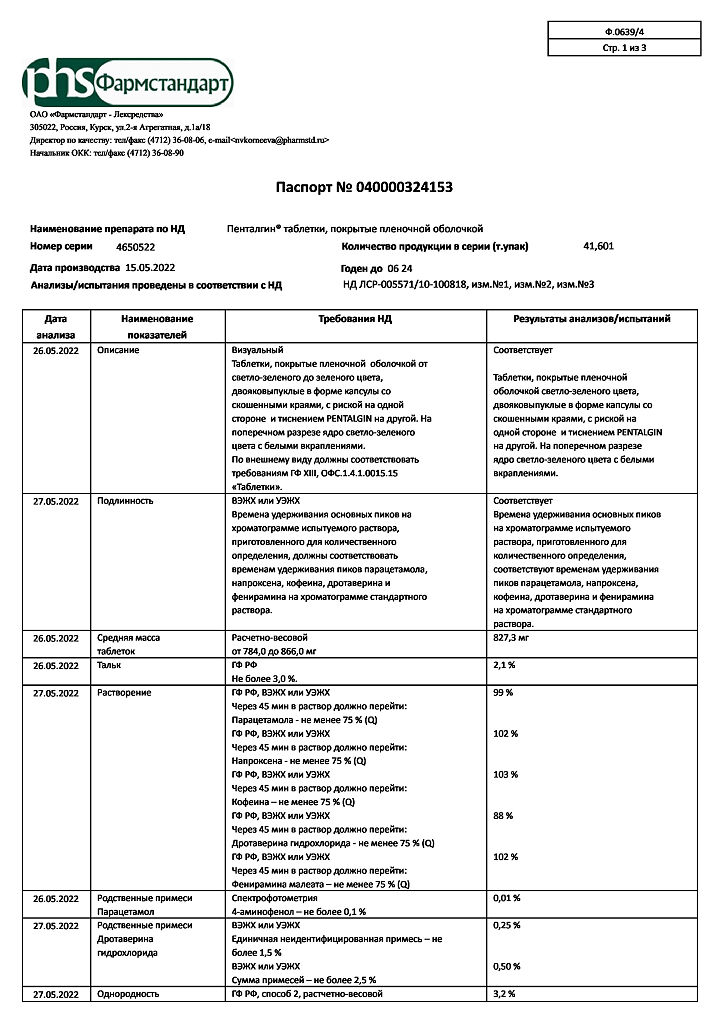
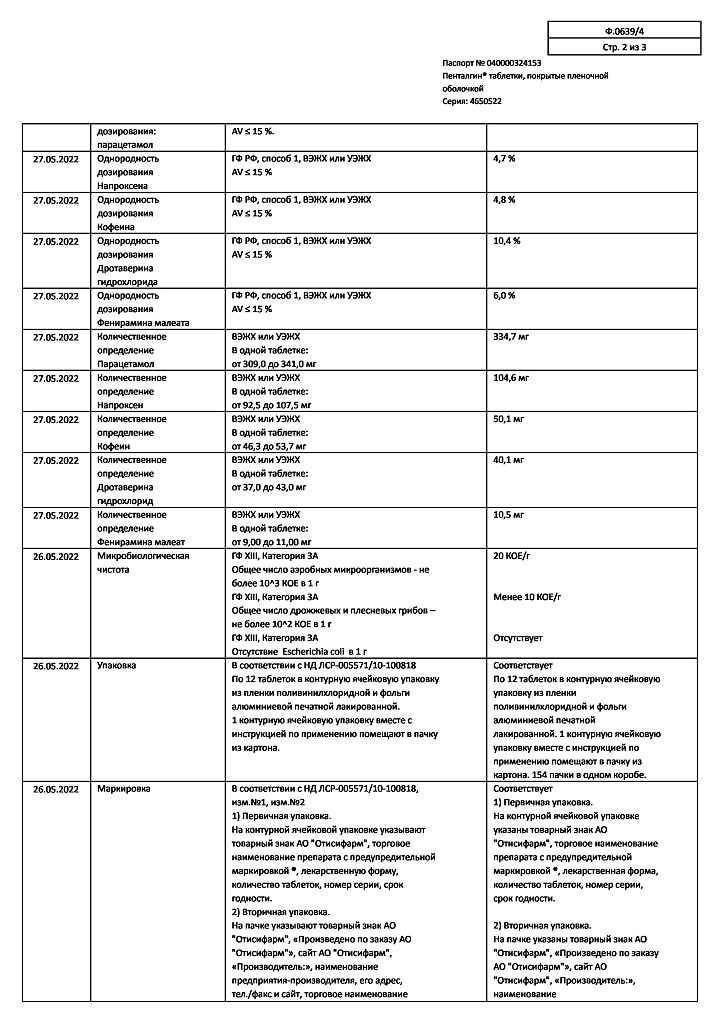
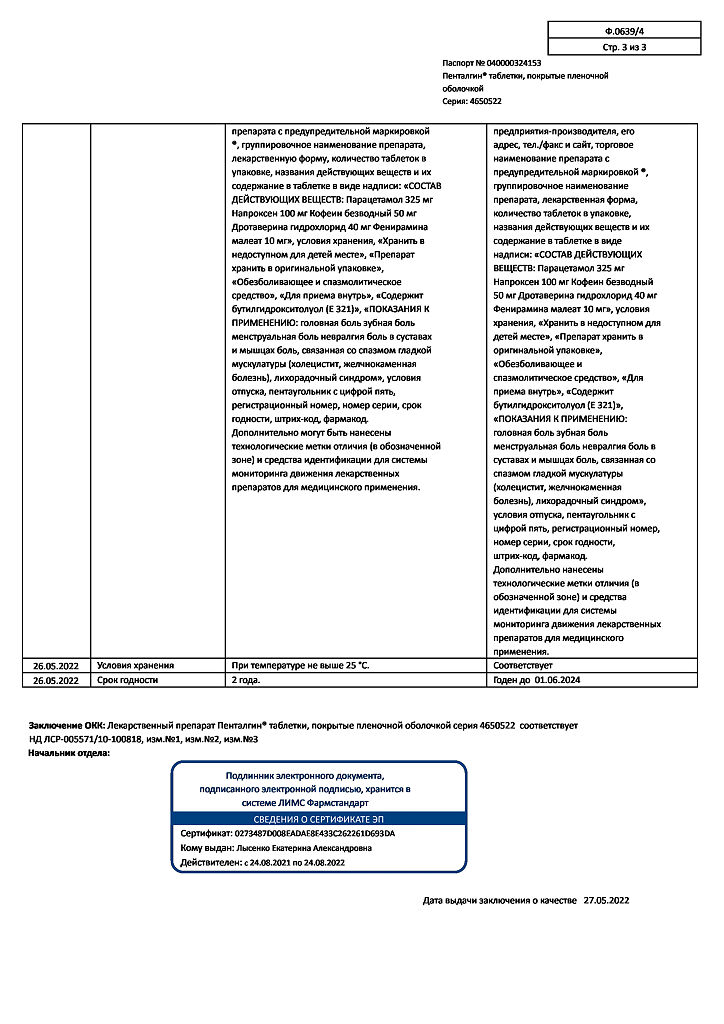
| Shelf life | 2 years |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 oC. Store out of the reach of children. |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | pills |
| Brand | Pharmstandard-Leksredstva |
Other forms…
Related products
Buy Pentalgin, 12 pcs. with delivery to USA, UK, Europe and over 120 other countries.