No products in the cart.
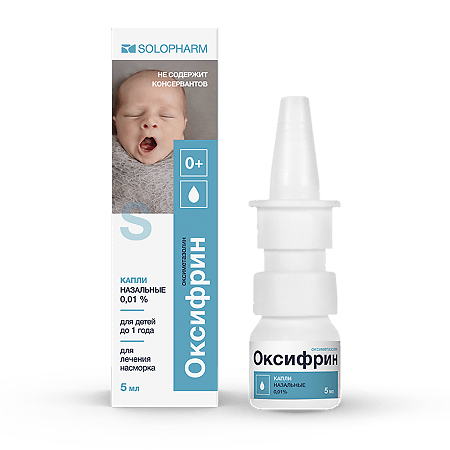
Oxyfrine, 0.01% drops 5 ml
€5.67 €4.73
Description
Pharmacodynamics:
Oxymetazoline belongs to the group of alpha-adrenergic stimulants for local use. It has a vasoconstrictor effect. When administered intranasally it reduces swelling of the mucous membrane of the upper airways and nasal secretions. It restores nasal breathing.
The reduction of mucous membrane edema of the nasal cavity restores aeration of the paranasal sinuses of the middle ear cavity which reduces the likelihood of bacterial complications (maxillary sinusitis otitis media).
In local intranasal application and therapeutic concentrations, it does not irritate and cause nasal mucosal hyperemia.
Oxymetazoline begins to act quickly within minutes. The duration of action is up to 12 hours.
Pharmacokinetics:
Oxymetazoline has no systemic action when administered topically intranasally. The half-life of oxymetazoline when administered intranasally is 35 h. 21% of oxymetazoline is excreted in the urine and about 11% in the feces.
Indications
Indications
treatment of acute respiratory diseases accompanied by a runny nose;
allergic rhinitis;
vasomotor rhinitis;
to restore drainage in case of inflammation of the paranasal sinuses, eustachitis, otitis media;
to eliminate swelling before diagnostic manipulations in the nasal passages
Pharmacological effect
Pharmacological effect
Pharmacodynamics:
Oxymetazoline belongs to the group of alpha-agonists for topical use. Has a vasoconstrictor effect. When administered intranasally, it reduces swelling of the mucous membrane of the upper respiratory tract and nasal discharge. Restores nasal breathing.
Elimination of swelling of the nasal mucosa helps restore aeration of the paranasal sinuses of the nasal cavity and the middle ear, which reduces the likelihood of bacterial complications (sinusitis, sinusitis, otitis media).
When applied locally and intranasally at therapeutic concentrations, it does not irritate or cause hyperemia of the nasal mucosa.
Oxymetazoline begins to act quickly within a few minutes. Duration of action is up to 12 hours.
Pharmacokinetics:
When administered locally intranasally, oxymetazoline does not have a systemic effect. The half-life of oxymetazoline when administered intranasally is 35 hours. 21% of oxymetazoline is excreted in the urine and about 11% in the feces.
Special instructions
Special instructions
Avoid getting the drug into your eyes.
To avoid the spread of infection, it is necessary to use the drug individually.
Impact on the ability to drive vehicles and machinery
After long-term use of cold remedies containing oxymetazoline in doses higher than recommended, a general effect on the cardiovascular system and central nervous system cannot be excluded.
In these cases, care should be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Oxymetazoline
Composition
Composition
1 ml of the drug contains:
Active substance:
Oxymetazoline hydrochloride 0.1 mg
Excipients:
Anhydrous citric acid 0.557 mg
Sodium citrate dihydrate 3.823 mg
Glycerol anhydrous 21, 165 mg
Water for injections up to 1 ml
Contraindications
Contraindications
hypersensitivity to the components of the drug;
atrophic (dry) rhinitis;
angle-closure glaucoma;
condition after transsphenoidal hypophysectomy;
surgical interventions on the dura mater (in history).
With caution
In patients suffering from diseases of the cardiovascular system (arterial hypertension, coronary heart disease, chronic heart failure, severe atherosclerosis, tachycardia, arrhythmias), carbohydrate metabolism disorders (diabetes mellitus), thyroid dysfunction (hyperthyroidism), pheochromocytoma, chronic renal failure, prostatic hyperplasia with clinical symptoms (urinary retention), increased intraocular pressure, porphyria, as well as in patients taking monoamine oxidase inhibitors during the previous 2 weeks and within 2 weeks after their discontinuation, tricyclic antidepressants, bromocriptine.
Side Effects
Side Effects
Burning or dryness of the mucous membranes of the nasal cavity, dryness of the mucous membranes of the mouth and throat; sneezing; an increase in the volume of secretions released from the nose; nosebleeds; after the effect of using the drug wears off, a feeling of “stuffiness” in the nose (reactive hyperemia).
Side effects caused by the systemic effect of the drug: increased blood pressure, headache, dizziness, palpitations, tachycardia, restlessness, anxiety, fatigue, drowsiness, sedation, irritability, sleep disturbance (in children), nausea, insomnia, exanthema, blurred vision (if in contact with the eyes), hallucinations, Quincke’s edema, itching, convulsions, respiratory arrest (in infants).
Long-term continuous use of vasoconstrictor drugs can lead to tachyphylaxis, atrophy of the nasal mucosa and recurrent swelling of the nasal mucosa (rhinitis medicamentosa).
If any of the side effects indicated in the instructions get worse or you notice any other side effects not listed in the instructions, tell your doctor.
Overdose
Overdose
In case of accidental ingestion of the drug or overdose, the following symptoms may appear: anxiety anxiety hallucinations convulsions decreased body temperature lethargy drowsiness coma constriction or dilation of the pupils fever sweating pallor cyanosis palpitations bradycardia arrhythmia cardiac arrest increased blood pressure decreased blood pressure nausea vomiting respiratory depression respiratory arrest.
Treatment: gastric lavage, intake of activated carbon (in case of accidental ingestion of the drug); symptomatic.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use after the expiration date.
Manufacturer
Manufacturer
Grotex LLC, Russia
Additional information
| Shelf life | 2 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At a temperature not higher than 25 ° C. Keep out of reach of children. |
| Manufacturer | Grotex Ltd, Russia |
| Medication form | nasal drops |
| Brand | Grotex Ltd |
Other forms…
Related products
Buy Oxyfrine, 0.01% drops 5 ml with delivery to USA, UK, Europe and over 120 other countries.