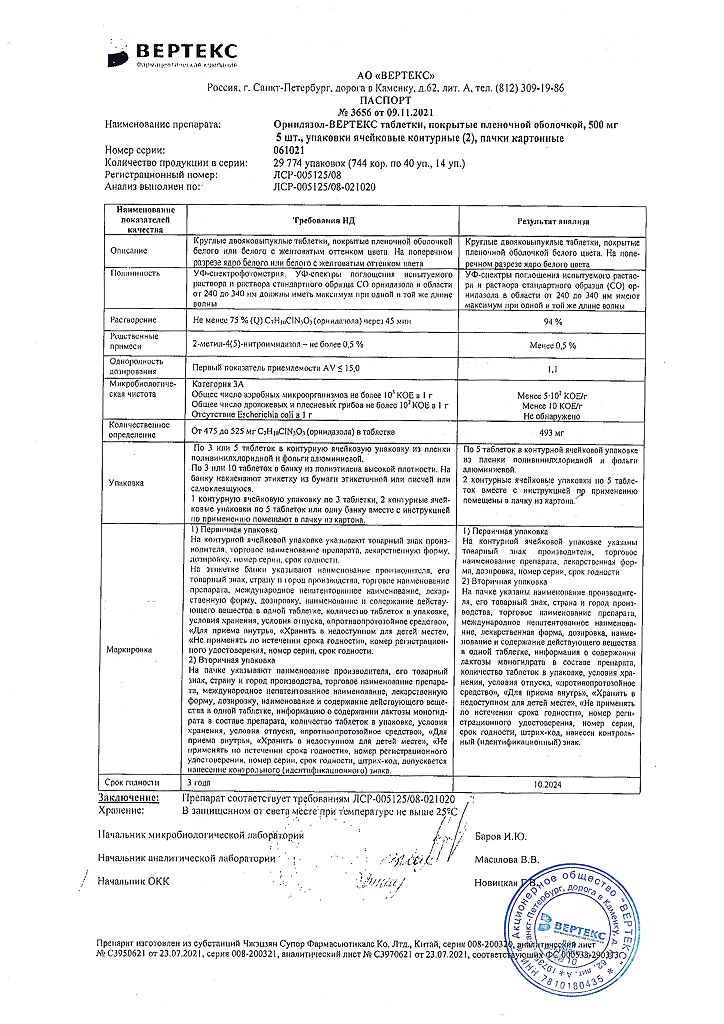
No products in the cart.
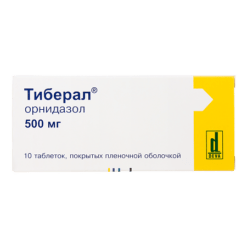
Ornidazole-Vertex, 500 mg 10 pcs
€8.12 €7.44
EAN: 4607003244248
SKU: 219701
Categories: Gynecology and Obstetrics, Medicine, Trichomoniasis and malaria
Description
Ornidazole is an antiprotozoal and antimicrobial drug, a derivative of 5-nitroimidazole. The mechanism of action consists in biochemical reduction of the 5-nitrogroup of ornidazole by intracellular transport proteins of anaerobic microorganisms and protozoa. The reduced 5-nitrogroup of ornidazole interacts with the DNA cells of microorganisms, inhibiting the synthesis of their nucleic acids, which leads to the death of the bacteria.
It is active against Trichomonas vaginalis, Gardnerella vaginalis, Giardia intestinalis, Entamoeba histolitica, Lamblia spp, as well as obligate anaerobes Bacteroides spp. (including Bacteroides fraglis, Bacteroides distasonis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides vulgatus), Fusobacterium spp, Veillonela spp., Prevotella (P.bivia, P.buccae, P.disiens), and some gram-positive microorganisms (Eubacterium spp., Clostridium). Aerobic microorganisms are not sensitive to ornidazole.
Pharmacokinetics
Gastrointestinal absorption is high, bioavailability is 90%. Binding to plasma proteins is less than 15%.
It is metabolized in the liver by hydroxylation, oxidation and glucuronidation. T1/2 is 12-14 hours.
It penetrates into breast milk and most tissues, passes through the HEB and the placenta. It is excreted as metabolites by kidneys (60-70%) and with feces (20-25%), about 5% of dose is excreted unchanged.
Indications
Indications
trichomoniasis;
amebiasis: amoebic dysentery, extraintestinal amebiasis (including amoebic liver abscess);
giardiasis;
prevention of infections caused by anaerobic bacteria during operations on the colon and gynecology.
Pharmacological effect
Pharmacological effect
Ornidazole is an antiprotozoal and antimicrobial drug, a derivative of 5-nitroimidazole. The mechanism of action is the biochemical reduction of the 5-nitro group of ornidazole by intracellular transport proteins of anaerobic microorganisms and protozoa. The reduced 5-nitro group of ornidazole interacts with the DNA cells of microorganisms, inhibiting the synthesis of their nucleic acids, which leads to the death of bacteria.
Active against Trichomonas vaginalis, Gardnerella vaginalis, Giardia intestinalis, Entamoeba histolitica, Lamblia spp., as well as obligate anaerobes Bacteroides spp. (including Bacteroides fraglis, Bacteroides distasonis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides vulgatus), Fusobacterium spp., Veillonela spp., Prevotella (P.bivia, P.buccae, P.disiens), and some gram-positive microorganisms (Eubacterium spp., Clostridium). Aerobic microorganisms are not sensitive to ornidazole.
Pharmacokinetics
Absorption from the gastrointestinal tract is high, bioavailability is 90%. Communication with plasma proteins is less than 15%.
Metabolized in the liver by hydroxylation, oxidation and glucuronidation. T1/2 – 12-14 hours.
Penetrates into breast milk and most tissues, passes through the blood-brain barrier and placenta. It is excreted in the form of metabolites by the kidneys (60-70%) and with feces (20-25%), about 5% of the dose is excreted unchanged.
Active ingredient
Active ingredient
Ornidazole
Composition
Composition
Active ingredient: ornidazole 500 mg;
Excipients:
lactose (milk sugar),
microcrystalline cellulose,
povidone (polyvinylpyrrolidone),
sodium lauryl sulfate,
calcium stearate,
colloidal silicon dioxide (Aerosil),
Primellose (croscarmellose sodium),
hypromellose (hydroxypropyl methylcellulose),
macrogol 4000 (polyethylene glycol 4000),
talc,
titanium dioxide
Contraindications
Contraindications
hypersensitivity;
pregnancy (first trimester);
lactation period;
due to the indivisibility of the dosage form, it is not prescribed to children weighing less than 12 kg.
With caution: central nervous system diseases (including epilepsy, multiple sclerosis), liver diseases, alcoholism, pregnancy, lactation.
Side Effects
Side Effects
Drowsiness, headache, dysfunction of the gastrointestinal tract (including nausea), dizziness, tremor, muscle rigidity, loss of coordination, convulsions, fatigue, temporary loss of consciousness, sensory or mixed peripheral neuropathy, perversion of taste, changes in the activity of liver enzymes, allergic reactions.
Interaction
Interaction
Enhances the effect of coumarin anticoagulants and prolongs the muscle relaxant effect of vecuronium bromide.
Compatible with ethanol (does not inhibit acetaldehyde dehydrogenase) unlike other imidazole derivatives (metronidazole).
Overdose
Overdose
Symptoms: epileptiform seizures, depression, peripheral neuritis.
Treatment: symptomatic (diazepam – for seizures).
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Vertex, Russia |
| Medication form | pills |
| Brand | Vertex |
Other forms…
Related products
Buy Ornidazole-Vertex, 500 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.