No products in the cart.
Omaron, tablets 90 pcs
€11.69 €9.74
Description
Combined medicine with pronounced antihypoxic, nootropic and vasodilator effect.
Piracetam activates metabolic processes in the brain by enhancing energy and protein metabolism, accelerating glucose utilization by cells and increasing their resistance to hypoxia;
improves inter-neuronal transmission in the central nervous system, improves regional blood flow in the ischemic area.
Cinnarizine is a selective blocker of “slow” calcium channels, reduces the entry of calcium ions into cells and reduces its content in the plasma membrane depot, reduces the tone of arteriolar smooth muscle,
reduces their response to biogenic vasoconstrictors (adrenaline, noradrenaline, dopamine, angiotensin II, vasopressin, serotonin).
It has a vasodilator effect (especially on brain vessels, increasing the antihypoxic effect of piracetam), without a significant effect on blood pressure.
It has moderate antihistamine activity, decreases the excitability of the vestibular system, reduces the tone of the sympathetic nervous system.
Increases elasticity of erythrocyte membranes, their ability to deform, reduces blood viscosity.
Absorption. After oral administration piracetam and cinnarizine are quickly and almost completely absorbed in the gastrointestinal tract. Bioavailability of piracetam is about 100%.
Maximum concentration (Cmax) of piracetam is reached 0.5-1 hour after intake. The maximum concentration of cinnarizine in plasma is reached after 1-3 hours. Bioavailability of cinnarizine increases in acidic environment.
Distribution. Piracetam does not bind with blood plasma proteins. The volume of distribution is about 0.6 l/kg.
Passes through the blood-brain and placental barriers, to all organs and tissues, as well as through filter membranes used in hemodialysis.
In animal studies it was found that piracetam selectively accumulates in cortical tissues, mainly in the frontal, parietal and occipital lobes, cerebellum and basal ganglia.
Cinnarizine. Binding with plasma proteins is 91%. In 1-4 hours after oral administration it is found in liver, kidneys, heart, lungs, spleen and brain.
Metabolism. Piracetam is virtually unmetabolized in the body.
Cinnarizine is actively and completely metabolized by dealkylation; the metabolic process begins 30 minutes after oral administration.
Excretion. Piracetam. More than 95% of the oral dose is eliminated unchanged by the kidneys by renal filtration within 30 hours.
Renal clearance of piracetam in healthy volunteers is 86 ml/min.
Half-life (T1/2) is 4-5 hours from blood plasma and 8.5 hours from cerebrospinal fluid.
In patients with renal insufficiency T1/2 is prolonged. In patients with hepatic insufficiency pharmacokinetics of piracetam does not change.
Cinnarizine is excreted from the body as metabolites (1/3 – by kidneys, 2/3 – through intestine), T1/2 – about 4 hours.
Indications
Indications
Diseases of the central nervous system, accompanied by a decrease in intellectual and mnestic functions.
As part of complex therapy: cerebrovascular insufficiency (cerebral atherosclerosis, recovery period after ischemic and hemorrhagic stroke);
post-intoxication or post-traumatic encephalopathy; depression; psychoorganic syndrome with a predominance of signs of asthenia and adynamia; vestibular disorders;
Meniere’s syndrome; delayed intellectual development in children; migraine prevention; prevention of kinetosis in adults and children.
Pharmacological effect
Pharmacological effect
A combined drug with a pronounced antihypoxic, nootropic and vasodilating effect.
Piracetam activates metabolic processes in the brain by enhancing energy and protein metabolism, accelerating the utilization of glucose by cells and increasing their resistance to hypoxia;
improves interneuronal transmission in the central nervous system, improves regional blood flow in the ischemic area.
Cinnarizine is a selective blocker of “slow” calcium channels, reduces the entry of calcium ions into cells and reduces its content in the plasma membrane depot, reduces the tone of the smooth muscles of arterioles,
reduces their response to biogenic vasoconstrictors (adrenaline, norepinephrine, dopamine, angiotensin II, vasopressin, serotonin).
It has a vasodilating effect (especially in relation to cerebral vessels, enhancing the antihypoxic effect of piracetam), without having a significant effect on blood pressure.
It exhibits moderate antihistamine activity, reduces the excitability of the vestibular apparatus, and lowers the tone of the sympathetic nervous system.
Increases the elasticity of red blood cell membranes, their ability to deform, and reduces blood viscosity.
Suction. After oral administration, piracetam and cinnarizine are quickly and almost completely absorbed from the gastrointestinal tract. The bioavailability of piracetam is about 100%.
The maximum concentration (Cmax) of piracetam is achieved 0.5-1 hour after administration. The maximum concentration of cinnarizine in plasma is achieved after 1-3 hours. The bioavailability of cinnarizine increases in an acidic environment.
Distribution. Piracetam does not bind to plasma proteins. The volume of distribution is approximately 0.6 l/kg.
Penetrates through the blood-brain and placental barriers, into all organs and tissues, as well as through filter membranes used in hemodialysis.
In animal studies, it was found that piracetam selectively accumulates in the tissues of the cerebral cortex, mainly in the frontal, parietal and occipital lobes, cerebellum and basal ganglia.
Cinnarizine. Plasma protein binding is 91%. 1-4 hours after ingestion it is found in the liver, kidneys, heart, lungs, spleen and brain.
Metabolism. Piracetam is practically not metabolized in the body.
Cinnarizine is actively and completely metabolized by dealkylation; the metabolic process begins 30 minutes after ingestion.
Excretion. Piracetam. More than 95% of the dose taken orally is excreted unchanged by the kidneys by renal filtration within 30 hours.
The renal clearance of piracetam in healthy volunteers is 86 ml/min.
The half-life (T1/2) is 4-5 hours from blood plasma and 8.5 hours from cerebrospinal fluid.
In patients with renal failure, T1/2 is prolonged. In patients with liver failure, the pharmacokinetics of piracetam does not change.
Cinnarizine is excreted from the body in the form of metabolites (1/3 – by the kidneys, 2/3 – through the intestines), T1/2 – about 4 hours.
Special instructions
Special instructions
With long-term use, monitoring of liver and kidney function is recommended (especially in patients with chronic renal failure).
During treatment, patients with arterial hypotension may experience a more significant decrease in blood pressure.
Drinking alcohol is not recommended during treatment.
The results of doping tests and allergic skin tests may be distorted; 4 days before the study, the drug should be discontinued.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Piracetam, Cinnarizine
Active components
Active components
Piracetam + Cinnarizine
Composition
Composition
Active ingredients:
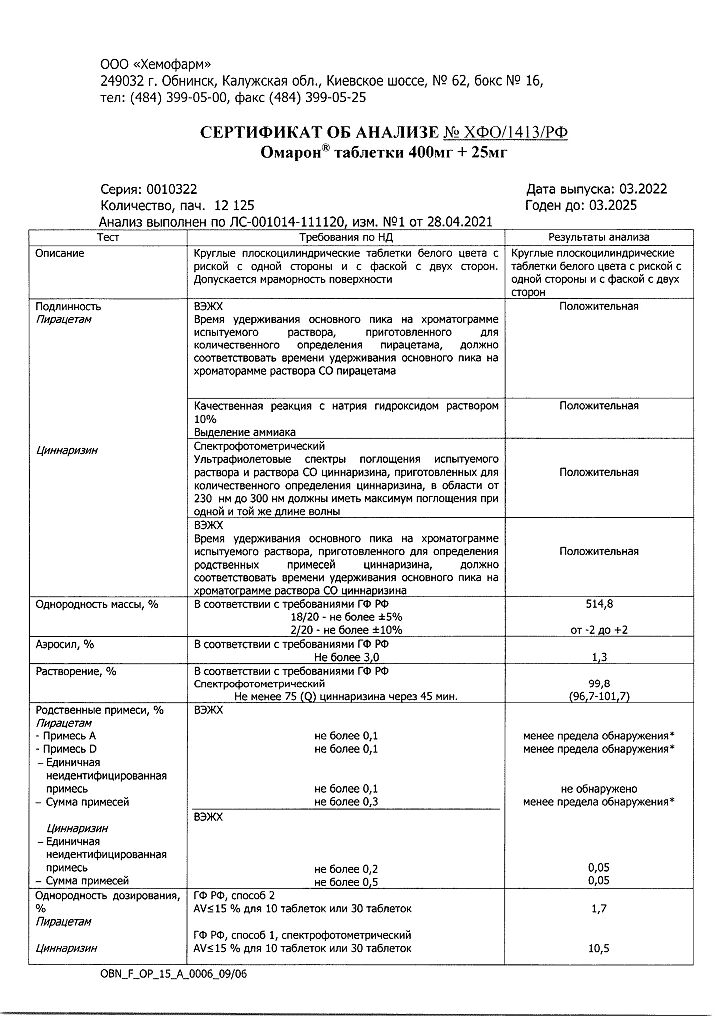
Piracetam 400 mg;
Cinnarizine 25 mg;
Excipients:
Lactose monohydrate 23.5 mg;
Magnesium hydroxycarbonate pentahydrate 46.8 mg;
Povidone (kollidon 30) 3.9 mg;
Colloidal silicon dioxide (Aerosil A-380) 5.2 mg,
Calcium stearate monohydrate 5.2 mg;
Crospovidone (kollidon CL-M) 10.4 mg.
Pregnancy
Pregnancy
The drug is contraindicated for use during pregnancy and lactation.
Contraindications
Contraindications
Hypersensitivity to the main and/or auxiliary components of the drug; severe liver failure; severe renal failure (creatinine clearance less than 20 ml/min); hemorrhagic stroke;
parkinsonism (including Parkinson’s disease); psychomotor agitation; Huntington’s disease; pregnancy; lactation period; children under 5 years of age; lactose intolerance, lactase deficiency, glucose-galactose malabsorption syndrome (the drug contains lactose).
With caution
Liver and/or kidney diseases, chronic renal failure (creatinine clearance 20-80 ml/min); increased intraocular pressure; porphyria; violation of hemostasis; extensive surgical interventions;
heavy bleeding; hyperthyroidism; epilepsy; severe cerebral atherosclerosis; tendency to neurotic reactions.
Side Effects
Side Effects
From the central and peripheral nervous system: motor disinhibition, irritability, drowsiness, depression, asthenia, headache. In isolated cases, dizziness, ataxia,
exacerbation of epilepsy, extrapyramidal disorders, tremor, imbalance, decreased ability to concentrate, insomnia, agitation, anxiety, hallucinations, increased sexuality.
From the cardiovascular system: decrease or increase in blood pressure.
From the digestive system: dyspeptic symptoms, feeling of dry mouth; in isolated cases – nausea, vomiting, diarrhea, abdominal pain, cholestatic jaundice.
From the skin: in isolated cases, dermatitis, itching, skin rash.
Metabolism: weight gain.
Allergic reactions: angioedema.
Other: increased sweating; in isolated cases – lupus-like syndrome, lichen planus.
Interaction
Interaction
With simultaneous use, it is possible to enhance the sedative effect of drugs that depress the activity of the central nervous system, as well as ethanol, nootropic and antihypertensive drugs.
Vasodilating agents enhance the effect of the drug.
Improves tolerability of antipsychotic drugs and tricyclic antidepressants.
When used simultaneously, piracetam enhances the central effects of thyroid hormones (tremor, anxiety, irritability, sleep disturbances are possible); may enhance the effect of oral anticoagulants.
Overdose
Overdose
Symptoms of overdose due mainly to the m-anticholinergic activity of cinnarizine include: disturbances of consciousness, vomiting, extrapyramidal symptoms, decreased blood pressure. After oral administration of piracetam at a dose of 75 g, bloody diarrhea and abdominal pain were observed.
Treatment: There is no specific antidote. In case of overdose, gastric lavage and administration of activated charcoal, symptomatic and supportive therapy are necessary. The effectiveness of hemodialysis for piracetam is 50-60%.
Recommendations for use
Recommendations for use
Inside, during or after meals.
Adults: 1-2 tablets 3 times a day for 1-3 months, depending on the severity of the disease. It is possible to carry out repeated courses of treatment – 2-3 times a year.
Children over 5 years old: 1-2 tablets 1-2 times a day. Do not use for more than 3 months.
For the prevention of kinetosis: in adults – 1 tablet, in children over 5 years old – 1/2 tablet 30 minutes before the start of the trip, with repeated doses (if necessary) every 6-8 hours.
Patients with impaired renal function: for chronic renal failure (creatinine clearance 20-80 ml/min) – 1 tablet 2 times a day.
Prescribing
Prescribing
Nootropic drug
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 ° C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Nizhpharm JSC, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Nizhpharm AO, Russia |
| Medication form | pills |
| Brand | Nizhpharm AO |
Other forms…
Related products
Buy Omaron, tablets 90 pcs with delivery to USA, UK, Europe and over 120 other countries.