No products in the cart.
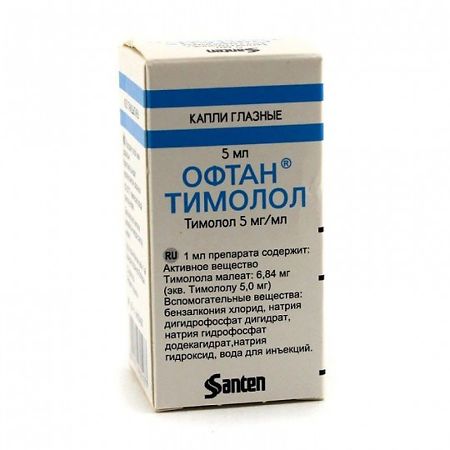
Oftan Timolol, eye drops 5 mg/ml 5 ml
€2.04 €1.00
Out of stock
(E-mail when Stock is available)
Description
The main active ingredient is timolol, a substance from the group of non-selective beta-adrenoblockers. Timolol blocks the beta-adrenoceptors without a parallel pronounced sympathomimetic effect. The mechanism of hypotensive action of Timolol eye drops is associated with inhibition of intraocular fluid (aqueous humor) production in the anterior chamber of the eye, as well as facilitation of outflow of aqueous humor from the eye chambers. The drug has no effect on the size of the pupil and accommodation capacity of the eye, therefore it does not affect visual acuity and does not worsen the quality of twilight vision.
The drug reduces both elevated and normal intraocular pressure regardless of whether the patient has glaucoma or not.
A pronounced hypotensive effect develops on the average 30 minutes after a single use of the eye drops and its maximum effect is 1-2 hours after instillation of the solution into the conjunctival sac; the pressure remains reduced for 24 hours after a single use of the medicine.
Timolol is well tolerated and causes a small number of side effects and when used as directed has almost no effect on heart rate.
Indications
Indications
To reduce intraocular pressure and prevent the development of complications in patients with increased ophthalmotonus and primary open-angle glaucoma, as well as secondary glaucoma (including post-traumatic, uveal and aphakic).
The drug can be prescribed in complex therapy of closed glaucoma (when prescribed with mitotics), as an auxiliary drug for congenital glaucoma or in case of acute increase in intraocular pressure.
Pharmacological effect
Pharmacological effect
The main active ingredient is timolol, a substance from the group of non-selective beta-blockers. Timolol blocks beta-adrenergic receptors without simultaneously producing a pronounced sympathomimetic effect. The mechanism of the hypotensive effect of Timolol eye drops is associated with inhibition of the production of intraocular fluid (aqueous humor) in the anterior chamber of the eye, as well as facilitating the outflow of aqueous humor from the chambers of the eye. The drug does not affect the size of the pupil and the ability of the eye to accommodate, therefore it does not impair visual acuity and does not impair the quality of twilight vision.
The drug reduces both elevated and normal intraocular pressure, regardless of the presence or absence of glaucoma in the patient.
A pronounced hypotensive effect develops on average 30 minutes after a single use of eye drops, its maximum occurs 1-2 hours from the moment of instillation of the solution into the conjunctival sac, the pressure remains reduced for 24 hours after a single use of the drug.
Timolol is well tolerated, causes a small number of side effects, and, when used according to the instructions, has virtually no effect on heart rate.
Special instructions
Special instructions
Visual impairment, dizziness and fatigue may occasionally occur when using Oftan Timolol eye drops.
It is recommended to monitor the effectiveness of the drug approximately 3-4 weeks after the start of therapy (no earlier than 1-2 weeks). With long-term use of timolol, the effect may weaken.
When used together with beta blockers, calcium channel blockers, an excessive decrease in blood pressure may occur. When using the drug Oftan Timolol, the function of tear secretion, the integrity of the cornea, and the field of vision should be monitored at least once every 6 months.
Oftan Timolol contains the preservative benzalkonium chloride, which can cause eye irritation, be absorbed by soft contact lenses, causing discoloration, and have adverse effects on eye tissue. Contact lenses should be removed before using the drug and, if necessary, reinserted no earlier than 15 minutes after instillation.
When transferring patients to timolol treatment, correction of refractive changes caused by previously used miotics may be required.
The drug Oftan Timolol is prescribed with caution to patients with impaired liver function, kidney function, or diabetes mellitus.
In case of upcoming surgery under general anesthesia, it is necessary to discontinue the drug 48 hours before surgery, because timolol enhances the effect of muscle relaxants and general anesthetics.
Do not use two different beta blockers in the same eye.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care must be taken when driving vehicles and when working with complex equipment that requires increased concentration, speed of psychomotor reactions and good vision (within 30 minutes after instillation into the eye), because the drug can lower blood pressure, cause fatigue and dizziness.
Active ingredient
Active ingredient
Timolol
Composition
Composition
Active ingredients:
Pregnancy
Pregnancy
There is no sufficient experience with the use of the drug during pregnancy and lactation, but it has been established that timolol penetrates the placental barrier and is excreted in breast milk. As prescribed by the attending physician, Oftan® Timolol can be used during pregnancy and breastfeeding in cases where the expected therapeutic effect for the mother justifies the potential risk for the fetus and child.
If the drug was used immediately before childbirth or during breastfeeding, then newborns should be closely monitored for several days after birth and during the entire period of treatment of nursing mothers with Oftan® Timolol.
Use in children
Due to the lack of data on effectiveness and safety, the use of the drug in children and adolescents under 18 years of age is contraindicated.
Contraindications
Contraindications
Timolol should not be prescribed for the treatment of patients under 18 years of age suffering from bronchial asthma, chronic obstructive pulmonary diseases, sinus bradycardia, atrioventricular block II – III degree, chronic heart failure in the stage of decompensation, cardiogenic shock, dystrophic changes in the cornea. Do not use if hypersensitivity to any of the components of the drug occurs.
The use of Timolol requires special caution in patients with arterial hypotension, sinoatrial blockades of degree II – III, atrophic rhinitis, pulmonary insufficiency, severe cerebrovascular accidents, diabetes mellitus, myasthenia gravis, thyrotoxic goiter, pheochromocytoma, Raynaud’s syndrome, during pregnancy or breastfeeding.
Simultaneous administration of other beta-blockers and psychoactive drugs that increase the release of epinephrine.
Side Effects
Side Effects
From the organ of vision: blurred vision, irritation and hyperemia of the conjunctiva, burning and itching of the eyes, lacrimation, swelling of the corneal epithelium, pinpoint superficial keratopathy, corneal hypersthesia, dry eye syndrome, blepharitis, conjunctivitis and keratitis. With long-term use, ptosis and, rarely, diplopia may develop. When performing fistulizing (penetrating) antiglaucoma operations, choroidal detachment may develop in the postoperative period.
From the cardiovascular system: bradycardia, bradyarrhythmia, decreased blood pressure, collapse, heart block, transient cerebrovascular accidents, exacerbation of chronic heart failure, chest pain.
From the digestive system: nausea, diarrhea.
From the respiratory system: nasal congestion, shortness of breath, bronchospasm, pulmonary insufficiency.
From the central nervous system and peripheral nervous system: headache, dizziness, weakness, confusion, hallucinations, insomnia, onirodynia, anxiety, mood changes, paresthesia.
From the skin: alopecia, psoriasis-like rashes and exacerbation of psoriasis.
From the genitourinary system: Peyronie’s disease, decreased potency.
Allergic reactions: generalized or local rash, itching.
Other: myasthenia gravis, tinnitus.
Interaction
Interaction
Combined use of Oftan Timolol with eye drops containing epinephrine may cause pupil dilation.
With the simultaneous use of eye drops containing epinephrine and pilocarpine, an increased reduction in intraocular pressure is possible.
Arterial hypotension and bradycardia may increase with simultaneous use of Oftan Timolol with calcium antagonists, reserpine and systemic beta-blockers.
CYP2D6 inhibitors such as quinidine and cimetidine may increase plasma concentrations of timolol.
Concomitant use with insulin or oral antidiabetic agents may lead to hypoglycemia.
Timolol enhances the effect of muscle relaxants, so it is necessary to discontinue the drug 48 hours before the planned surgical intervention under general anesthesia. These data may also apply to medications that were used shortly before.
Overdose
Overdose
Symptoms: possible development of systemic effects characteristic of beta-blockers (dizziness, headache, arrhythmia, bradycardia, bronchospasm, nausea and vomiting).
Treatment: immediately rinse eyes with water or saline, symptomatic therapy.
Storage conditions
Storage conditions
At 15–25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Santen JSC, Finland
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At 15-25 °C |
| Manufacturer | Santen AO, Finland |
| Medication form | eye drops |
| Brand | Santen AO |
Related products
Buy Oftan Timolol, eye drops 5 mg/ml 5 ml with delivery to USA, UK, Europe and over 120 other countries.