No products in the cart.
Ocumed, eye drops 0.5% 10 ml
€3.70 €3.08
Description
A nonselective beta-adrenoblocker for topical use in ophthalmology. It has no intrinsic sympathomimetic and membranostabilizing activity.
The drug reduces both normal and elevated intraocular pressure by reducing the formation of intraocular fluid. It does not influence the pupil size and accommodation.
The action of the drug is seen 20 minutes after injection into the conjunctival sac. The maximum decrease of intraocular pressure occurs within 1-2 hours and lasts for 24 hours.
Indications
Indications
– increased intraocular pressure (ophthalmohypertension);
– open-angle glaucoma;
– secondary glaucoma;
– to reduce intraocular pressure in angle-closure glaucoma (as an additional agent in combination with miotics);
– congenital glaucoma (if other therapeutic measures are insufficient).
Pharmacological effect
Pharmacological effect
Non-selective beta-blocker for local use in ophthalmology. It does not have internal sympathomimetic and membrane-stabilizing activity.
The drug reduces both normal and elevated intraocular pressure by reducing the formation of intraocular fluid. Does not affect pupil size and accommodation.
The effect of the drug appears 20 minutes after instillation into the conjunctival sac. The maximum decrease in intraocular pressure develops after 1-2 hours and persists for 24 hours.
Special instructions
Special instructions
The patient should be warned about the need to regularly measure intraocular pressure and examine the cornea, as well as the need to stop using the drug and consult a doctor if side effects occur.
When wearing soft contact lenses, you should not use Okumed eye drops, because the preservative they contain can be deposited in soft contact lenses and have an adverse effect on eye tissue.
Hard contact lenses should be removed before instillation of the drug and reinserted after 15 minutes.
When transferring patients to treatment with Okumed, refractive error correction may be necessary (after the disappearance of the effects of previously used miotics).
Okumed must be discontinued 48 hours before surgery using general anesthesia.
Okumed should not be used simultaneously with antipsychotic (neuroleptics) and anxiolytic (tranquilizers) drugs.
During the period of use of the drug, it is not recommended to drink alcohol (a sharp decrease in blood pressure is possible).
Use in pediatrics
There is no sufficient experience with the use of the drug in children, so the drug can be prescribed only in cases where the expected benefits of therapy outweigh the potential risk of side effects.
Impact on the ability to drive vehicles and operate machinery
Immediately after using the drug, vision clarity and psychomotor reactions may decrease, which may reduce the ability to engage in potentially hazardous activities that require increased attention (especially when consuming alcohol at the same time).
Active ingredient
Active ingredient
Timolol
Composition
Composition
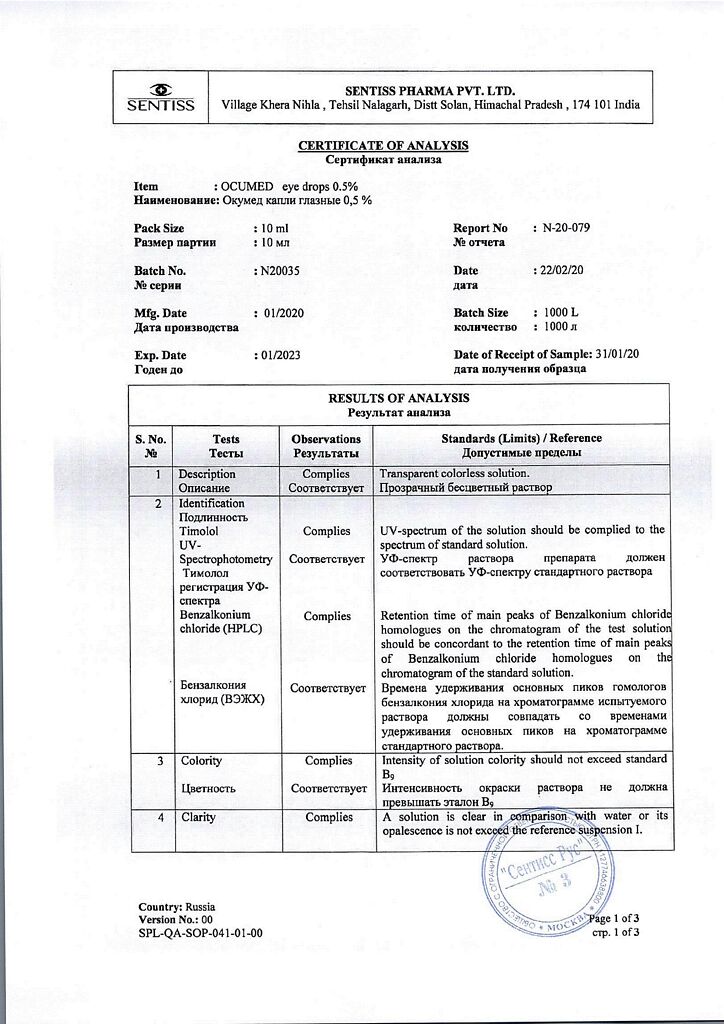
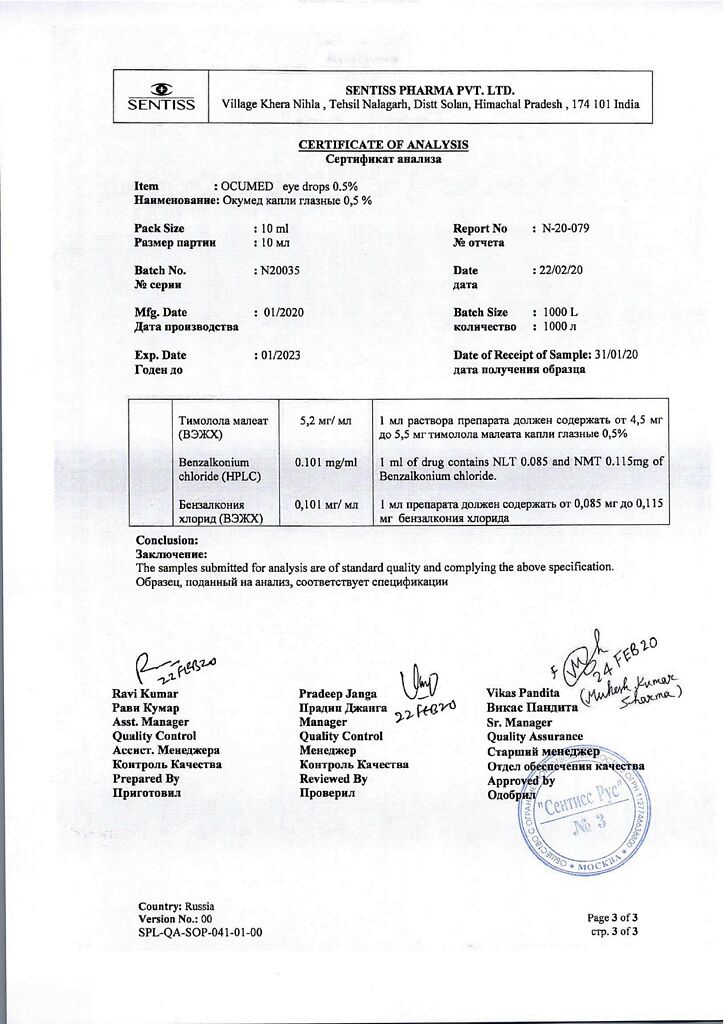
Timolol (maleate form) – 5 mg
Contraindications
Contraindications
– bronchial asthma or other severe chronic obstructive respiratory diseases;
– sinus bradycardia;
– AV block of II and III degrees;
– acute heart failure;
— severe chronic heart failure (III and IV functional class according to the NYHA classification);
– cardiogenic shock;
– allergic reactions with generalized skin rashes;
– atrophic rhinitis;
— dystrophic diseases of the cornea;
– neonatal period;
– hypersensitivity to the components of the drug.
The drug is prescribed with caution to patients with pulmonary insufficiency, pulmonary emphysema, severe cerebrovascular insufficiency, heart failure (functional class I and II according to the NYHA classification), sinoatrial blockade, arterial hypertension, diabetes mellitus, hypoglycemia, thyrotoxicosis, myasthenia gravis, Reye’s syndrome, while prescribing other beta-blockers, as well as children.
Side Effects
Side Effects
From the organ of vision: irritation and hyperemia of the conjunctiva, skin of the eyelids, burning and itching in the eyes, lacrimation or decreased tearing, photophobia, swelling of the corneal epithelium, pinpoint superficial keratopathy, corneal hypoesthesia, diplopia, ptosis, dry eyes, short-term impairment of visual acuity, blepharitis, conjunctivitis, keratitis. When performing surgical interventions for glaucoma, choroidal detachment may develop in the postoperative period.
From the cardiovascular system: chest pain, heart failure, bradycardia, bradyarrhythmia, decreased blood pressure, collapse, AV block, cardiac arrest, transient cerebrovascular accident.
From the respiratory system: rhinitis, shortness of breath, bronchospasm, pulmonary failure.
From the central nervous system and peripheral nervous system: headache, dizziness, weakness, depression, paresthesia, myasthenia, drowsiness, hallucinations, ringing in the ears, slow psychomotor reaction.
From the digestive system: nausea, vomiting, diarrhea.
Allergic reactions: urticaria, eczema.
Other: nosebleeds, decreased potency, alopecia.
Interaction
Interaction
The combined use of Okumed with eye drops containing epinephrine may cause pupil dilation.
The decrease in intraocular pressure is enhanced by the simultaneous use of eye drops containing epinephrine and pilocarpine. You should not put two beta-blockers in your eyes.
A decrease in blood pressure and a slowdown in heart rate may increase when the drug is used together with slow calcium channel blockers, reserpine and other beta-blockers.
Concomitant use with insulin or oral antidiabetic agents may lead to hypoglycemia.
Timolol enhances the effect of peripheral muscle relaxants (the drug must be discontinued 48 hours before the planned surgery using general anesthesia).
Overdose
Overdose
Symptoms: due to local absorption, systemic effects characteristic of beta-blockers (dizziness, headache, arrhythmia, bradycardia, bronchospasm, nausea, vomiting) may develop.
Treatment: Immediately rinse eyes with water or saline solution. Symptomatic therapy is carried out.
Storage conditions
Storage conditions
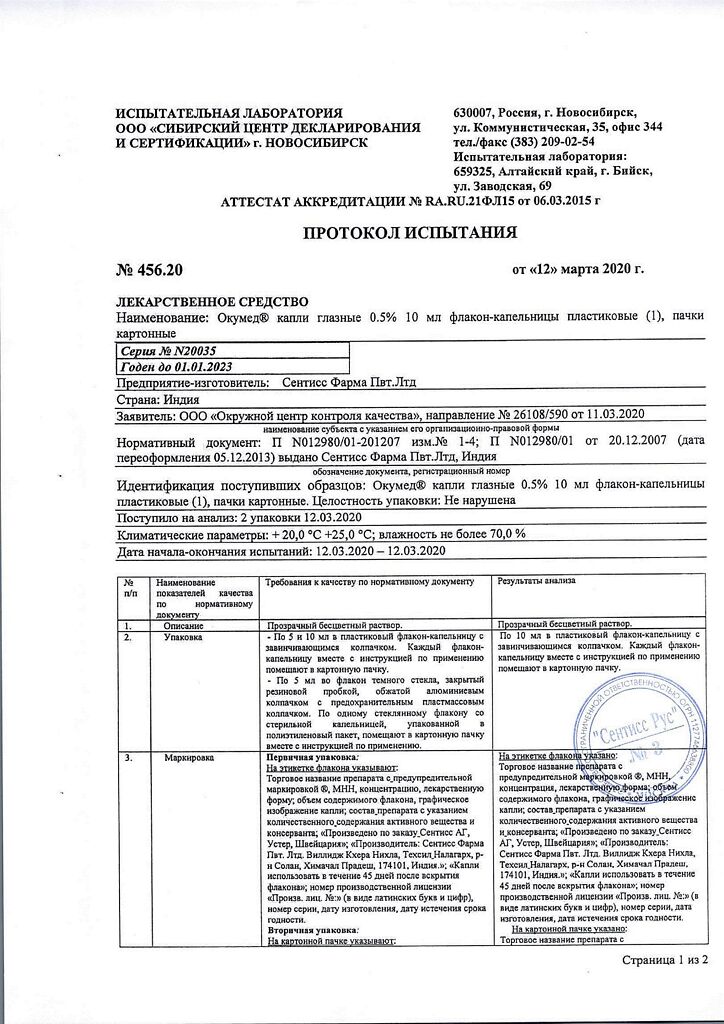
The drug should be stored out of the reach of children, protected from light at a temperature not exceeding 25°C; do not freeze
Shelf life
Shelf life
2 years.
Once opened, the bottle must be used within 45 days.
Manufacturer
Manufacturer
Sentiss Pharma Pvt.Ltd, India
Additional information
| Shelf life | 2 years. After opening the bottle must be used within 45 days. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, protected from light at a temperature not exceeding 25°C; do not freeze |
| Manufacturer | Sentiss Pharma Pvt.Ltd, India |
| Medication form | eye drops |
| Brand | Sentiss Pharma Pvt.Ltd |
Other forms…
Related products
Buy Ocumed, eye drops 0.5% 10 ml with delivery to USA, UK, Europe and over 120 other countries.