No products in the cart.
Nurofen 12+, 200 mg 12 pcs
€5.04 €4.48
Out of stock
(E-mail when Stock is available)
Description
Pharmacodynamics.
Ibuprofen is an NSAID, the action of which is associated with inhibition of prostaglandin synthesis. Ibuprofen has analgesic, antipyretic and anti-inflammatory effects. It has been clinically proven that when treating pain, ibuprofen tablets in the form of ibuprofen sodium salt begin to work significantly faster compared to ibuprofen in the form of acid.
Ibuprofen at a dose of 400 mg provides pain relief for up to 8 hours. In a study of toothache treatment, after using 2 tablets of Nurofen 12+, compared to placebo, a significant decrease in pain was felt in 15 minutes.
In this study, significantly more patients experienced a significant reduction in pain intensity after administration of 2 Nurofen 12+ tablets compared to 2 500 mg paracetamol tablets. Also in these patients a significant decrease of pain intensity and a significant decrease of pain severity within 6 hours compared to the use of paracetamol was found.
The experimental data indicate that ibuprofen can competitively inhibit the effect of low-dose acetylsalicylic acid on platelet aggregation when these drugs are used simultaneously.
Some pharmacodynamic studies indicate that when single doses of ibuprofen 400 mg were administered within 8 h before or within 30 min after administration of immediate-release acetylsalicylic acid (81 mg), a decrease in the effect of acetylsalicylic acid on thromboxane formation or platelet aggregation was observed.
While there is uncertainty regarding the extrapolation of these data to the clinical situation, the possibility cannot be excluded that regular long-term use of ibuprofen may reduce the cardioprotective effect of low-dose acetylsalicylic acid. With non-systemic use of ibuprofen, this clinically significant effect is considered unlikely.
Pharmacokinetics. When administered orally, ibuprofen is rapidly absorbed in the digestive tract and reaches Cmax in plasma after 45 min when used on an empty stomach. After using ibuprofen with meals Cmax in plasma is determined after 1-2 hours. T½ of ibuprofen is almost 2 hours.
After the use of Nurofen 12+ tablets Cmax in plasma is reached 35 min after the use of the drug on an empty stomach.
Ibuprofen actively (90%) binds to plasma proteins, slowly penetrates into the synovial cavities, where its concentration may remain high, while the plasma concentration decreases.
The metabolism of ibuprofen occurs in the liver. Ibuprofen is rapidly and completely eliminated from the body. More than 90% of the dose is excreted by the kidneys as metabolites and their compounds.
It should be taken into account that bioavailability of ibuprofen sodium salt is significantly higher and the effect occurs 2 times faster than when using conventional ibuprofen tablets.
There are no differences in pharmacokinetics depending on the age of the patient.
.
Indications
Indications
Symptomatic treatment of mild to moderate pain, in particular headaches, migraines, back pain, neuralgia, muscle pain, rheumatic pain, menstrual pain, toothache.
symptomatic treatment of symptoms of colds, flu and fever.
Pharmacological effect
Pharmacological effect
pharmacodynamics.
Ibuprofen is an NSAID whose action is associated with a slowdown in the synthesis of prostaglandins. Ibuprofen has analgesic, antipyretic and anti-inflammatory effects. Ibuprofen tablets in the ibuprofen sodium salt form have been clinically proven to work significantly faster in the treatment of pain compared to the ibuprofen acid form.
When using ibuprofen at a dose of 400 mg, pain relief lasts up to 8 hours. During a study of the treatment of toothache, after using 2 tablets of Nurofen 12+ compared to placebo, a significant reduction in pain was felt after 15 minutes.
In this study, significantly more patients experienced a significant reduction in pain after taking 2 tablets of Nurofen 12+ compared to taking 2 tablets of paracetamol 500 mg. Also, these patients showed a significant decrease in pain intensity and a significant decrease in pain severity within 6 hours compared to the use of paracetamol.
Experimental evidence suggests that ibuprofen may competitively inhibit the effect of low-dose acetylsalicylic acid on platelet aggregation when these drugs are administered concomitantly.
Some pharmacodynamic studies show that when single doses of ibuprofen 400 mg were administered within 8 hours before or within 30 minutes after administration of immediate-release acetylsalicylic acid (81 mg), a decrease in the effect of acetylsalicylic acid on thromboxane formation or platelet aggregation was observed.
Although there is uncertainty regarding the extrapolation of these data to the clinical situation, the possibility that regular long-term use of ibuprofen may reduce the cardioprotective effect of low-dose acetylsalicylic acid cannot be ruled out. With unsystematic use of ibuprofen, such a clinically significant effect is considered unlikely.
Pharmacokinetics. When administered orally, ibuprofen is rapidly absorbed from the digestive tract and reaches Cmax in the blood plasma after 45 minutes when administered on an empty stomach. After using ibuprofen with meals, Cmax in blood plasma is determined after 1–2 hours. T½ of ibuprofen is almost 2 hours.
Moreover, after using Nurofen 12+ tablets, Cmax in blood plasma is achieved 35 minutes after taking the drug on an empty stomach.
Ibuprofen actively (90%) binds to blood plasma proteins and slowly penetrates into the synovial cavities, where its concentration can remain high, while the concentration in the blood plasma decreases.
Metabolism of ibuprofen occurs in the liver. Ibuprofen is quickly and completely eliminated from the body. More than 90% of the dose is excreted by the kidneys in the form of metabolites and their compounds.
It should be borne in mind that the bioavailability of ibuprofen sodium salt is much higher and the effect occurs 2 times faster than when using regular ibuprofen tablets.
There are no differences in pharmacokinetics depending on the age of the patient.
Special instructions
Special instructions
Side effects that occur following the use of ibuprofen and NSAIDs in general can be reduced by using the minimum effective dose needed to treat symptoms for a short period of time.
Effects on the respiratory system. Bronchospasm may occur in patients with a history of asthma or allergic diseases.
Other NSAIDs. Concomitant use of ibuprofen with other NSAIDs, including selective COX-2 inhibitors, increases the risk of adverse reactions and should therefore be avoided.
Systemic lupus erythematosus and mixed connective tissue disease. Ibuprofen should be used with caution in cases of systemic lupus erythematosus and mixed connective tissue disease due to the increased risk of aseptic meningitis.
Effect on the kidneys. Long-term use of NSAIDs can lead to a dose-dependent decrease in prostaglandin synthesis and provoke the development of renal failure. A high risk of this reaction is observed in patients with impaired renal function, cardiac disorders, impaired liver function, patients taking diuretics, and elderly patients. In such patients, renal function must be monitored.
Effect on the liver. Liver dysfunction.
Effect on the cardiovascular and cerebrovascular system. Patients with hypertension and/or a history of mild to moderate congestive heart failure should initiate treatment with caution (consult a physician) as cases of fluid retention, hypertension, and edema have been reported with ibuprofen, as with other NSAIDs.
Clinical trial and epidemiological data suggest that the use of ibuprofen, especially at high doses (2400 mg/day), as well as long-term treatment, may be associated with an increased risk of arterial thrombotic complications (eg myocardial infarction or stroke). In general, data from epidemiological studies do not suggest that low-dose ibuprofen (eg, ≤1200 mg/day) is associated with an increased risk of arterial thrombotic complications.
Patients with uncontrolled hypertension, congestive heart failure (NYHA class II–III), known coronary artery disease, peripheral arterial disease, and/or cerebrovascular disease should be treated with ibuprofen only after careful clinical evaluation. High doses (2400 mg/day) should be avoided.
In patients with risk factors for cardiovascular complications (such as hypertension, hyperlipidemia, diabetes mellitus, smoking), long-term treatment with NSAIDs, especially if high doses of ibuprofen (2400 mg/day) are required, should be prescribed only after careful analysis.
Effect on the gastrointestinal tract. NSAIDs should be used with caution in patients with chronic inflammatory bowel diseases (ulcerative colitis, Crohn’s disease), as these conditions may be exacerbated. Cases of gastrointestinal bleeding, ulcer perforation, sometimes fatal, have been reported that occurred at any stage of NSAID treatment, regardless of the presence of warning symptoms or a history of severe gastrointestinal disorders.
The risk of gastrointestinal bleeding and ulcer perforation increases with increasing doses of NSAIDs, in patients with a history of ulcers, especially if complicated by bleeding or perforation, and in elderly patients. These patients should begin treatment with minimal doses. Caution should be exercised when treating patients receiving concomitant medications that increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants (eg, warfarin), selective serotonin reuptake inhibitors, or antiplatelet agents (eg, acetylsalicylic acid). During long-term treatment for these patients, as well as for patients who require concomitant use of low doses of acetylsalicylic acid or other drugs that increase gastrointestinal risk, the physician should consider the advisability of combination therapy with misoprostol or proton pump inhibitors.
Patients with a history of gastrointestinal disorders, especially the elderly, should be informed of any unusual gastrointestinal symptoms (mainly bleeding), especially gastrointestinal bleeding at the beginning of treatment. In the event of gastrointestinal bleeding or ulceration in patients receiving ibuprofen, treatment should be discontinued immediately.
Impact on the skin and subcutaneous tissue. Very rarely, severe forms of skin reactions may occur while taking NSAIDs, including exfoliative dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis. The highest risk of such reactions occurs in the early stages of therapy, in most cases the onset of such reactions occurs during the first month of treatment. Ibuprofen should be discontinued at the first sign of skin rash, pathological changes in the mucous membranes, or any other signs of hypersensitivity.
Effect on fertility in women. There is limited evidence that drugs that inhibit COX/prostaglandin synthesis may impair fertility in women by affecting ovulation. This effect is reversible when therapy is discontinued.
Each tablet contains approximately 24.3 mg (1.06 mmol) sodium, which should be taken into account when prescribing the drug to patients who are indicated for a low sodium diet.
Use during pregnancy and breastfeeding. Suppression of prostaglandin synthesis may negatively affect pregnancy and/or embryonic/fetal development. Data from epidemiological studies indicate an increased risk of miscarriage, congenital heart defects, and gastroschisis following the use of prostaglandin synthesis inhibitors in early pregnancy.
The risk is believed to increase with increasing dose and duration of therapy. The use of ibuprofen should be avoided during the first and second trimesters of pregnancy unless the potential benefit to the patient outweighs the potential risk to the fetus. If ibuprofen is used by a woman who is trying to become pregnant or during the first or second trimester of pregnancy, the lowest effective dose should be used for the shortest period of time possible.
During the third trimester of pregnancy, with the use of any prostaglandin synthesis inhibitors, effects on the fetus such as cardiopulmonary toxicity (premature closure of the fetal ductus arteriosus with pulmonary hypertension) and impaired renal function, which can progress to renal failure with the manifestation of oligohydramnios, are possible.
Ibuprofen is contraindicated in the third trimester of pregnancy due to the possibility of inhibition of uterine contractility, which can lead to an increase in the duration of labor with a possible increase in bleeding time in mother and child, even when using very low doses.
In limited studies, ibuprofen has been detected in breast milk at very low concentrations. It is unlikely to have a negative effect on a breastfed infant. NSAIDs are not recommended for use during breastfeeding.
Children. The drug is used in children over 12 years of age.
The ability to influence reaction speed when driving vehicles and other mechanisms. Provided that recommendations regarding dosage and duration of treatment are followed, the drug’s effect on the ability to drive vehicles or use other machinery is not expected.
But patients who experience dizziness, drowsiness, disorientation, or visual disturbances while taking NSAIDs should not drive or operate machinery.
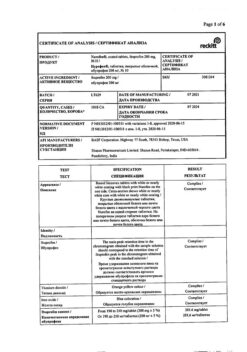
Active ingredient
Active ingredient
Ibuprofen
Composition
Composition
Active ingredients:
ibuprofen 200 mg in the form of ibuprofen sodium salt.
Excipients: croscarmellose sodium, xylitol (E 967), microcrystalline cellulose, magnesium stearate, colloidal anhydrous silicon dioxide, carmellose sodium, talc, acacia, sucrose, titanium dioxide (E 171), macrogol 6000, printing ink.
Contraindications
Contraindications
Individual hypersensitivity to ibuprofen or other components of the drug.
History of hypersensitivity reactions (eg asthma, rhinitis, angioedema or urticaria) after the use of ibuprofen, acetylsalicylic acid or other NSAIDs.
Gastric and/or duodenal ulcer or gastrointestinal bleeding in the acute stage or in history (2 or more severe episodes of confirmed ulcer or bleeding).
History of gastrointestinal bleeding and perforation associated with taking NSAIDs.
Severe liver dysfunction, severe renal impairment, severe heart failure (NYHA class IV).
Cerebrovascular or other bleeding.
Hematopoiesis or blood clotting disorders.
Glucose-galactose malabsorption syndrome or sucrose-isomaltose deficiency, fructose intolerance.
Last trimester of pregnancy.
Side Effects
Side Effects
Adverse reactions associated with the use of ibuprofen are classified by organ system and frequency. The frequency is determined as follows: very often (≥1/10); often (≥1/100 and <1/10); uncommon (≥1/1000 and <1/100); rare (≥1/10,000 and <1/1000); very rare (< 1/10,000), frequency unknown (frequency cannot be estimated from available data). In each frequency group, adverse reactions are presented by decreasing severity.
Data from clinical studies suggest that the use of ibuprofen, especially at high doses (2400 mg/day), may be associated with an increased risk of arterial thrombotic complications (eg, myocardial infarction or stroke).
From the blood and lymphatic system: very rarely – hematopoietic disorders1.
From the immune system: rarely – hypersensitivity reactions accompanied by urticaria and itching2; very rarely – severe hypersensitivity reactions, symptoms of which may include swelling of the face, tongue and larynx, shortness of breath, tachycardia, hypotension (anaphylaxis, angioedema or severe shock)2.
From the nervous system: infrequently – headache; very rarely – aseptic meningitis3; frequency unknown – paresthesia, drowsiness.
From the cardiovascular system: frequency unknown – heart failure, edema4.
From the vascular system: frequency unknown – AG4.
From the respiratory tract and mediastinal organs: frequency unknown – airway reactivity, including asthma, bronchospasm or shortness of breath2.
From the digestive tract: infrequently – abdominal pain, nausea, dyspepsia5; rarely – diarrhea, flatulence, constipation, vomiting; very rarely – peptic ulcer of the stomach and duodenum, gastrointestinal perforation or gastrointestinal bleeding, melena, hematemesis, sometimes fatal, ulcerative stomatitis, gastritis; frequency unknown – exacerbation of colitis and Crohn’s disease6.
From the liver: very rarely – impaired liver function; frequency unknown – with long-term treatment, hepatitis and jaundice may occur.
From the skin and subcutaneous tissue: uncommon – skin rash2; very rarely – bullous reactions, including Stevens-Johnson syndrome, erythema multiforme and toxic epidermal necrolysis2; frequency unknown – photosensitivity.
From the kidneys and urinary system: very rarely – acute renal dysfunction7; frequency unknown – renal failure, nephrotoxicity, including interstitial nephritis and nephrotic syndrome.
From the psyche: frequency unknown – only with long-term use: depression, hallucinations, confusion.
On the part of the organ of vision: frequency is unknown – with long-term treatment, visual impairment and optic neuritis may occur.
On the part of the hearing organ: frequency unknown – with long-term treatment, ringing in the ears and dizziness are possible.
Laboratory tests: very rarely – decreased hemoglobin levels.
Notes.
1 Includes anemia, leukopenia, thrombocytopenia, pancytopenia and agranulocytosis. The first signs of such disorders are fever, sore throat, superficial mouth ulcers, flu-like symptoms, severe exhaustion, bleeding and bruises of unknown etiology.
2 Hypersensitivity reactions may include: nonspecific allergic reactions and anaphylaxis; airway reactivity, including asthma, exacerbation of asthma, bronchospasm and shortness of breath, or various forms of skin reactions, including pruritus, urticaria, purpura, angioedema and, less commonly, exfoliative and bullous dermatosis, including toxic epidermal necrolysis, Stevens-Johnson syndrome and erythema multiforme.
3 The pathogenic mechanism of drug-induced aseptic meningitis is unclear. Available data on aseptic meningitis associated with NSAIDs indicate a hypersensitivity reaction (given the temporal relationship with the drug and the disappearance of symptoms after discontinuation of the drug).
In patients with autoimmune diseases (systemic lupus erythematosus and mixed connective tissue disease), isolated cases of symptoms of aseptic meningitis (stiff neck, headache, nausea, vomiting, fever and loss of consciousness) have been observed.
4 Clinical trial and epidemiological data suggest that the use of ibuprofen (especially at high doses of 2400 mg/day) and during long-term treatment may be associated with a slightly increased risk of arterial thrombotic complications (eg, myocardial infarction or stroke).
5 Most often, adverse reactions developed from the gastrointestinal tract.
6 See SPECIAL INSTRUCTIONS.
7 Especially with long-term use of NSAIDs in combination with an increase in urea levels in the blood plasma and the appearance of edema. Also includes papillonecrosis.
Interaction
Interaction
Ibuprofen, like other NSAIDs, should not be used in combination with:
acetylsalicylic acid, since this increases the risk of adverse reactions, unless acetylsalicylic acid (dose not exceeding 75 mg/day) was prescribed by a doctor;
other NSAIDs, including selective COX-2 inhibitors: the simultaneous use of two or more NSAIDs should be avoided as this increases the risk of adverse reactions.
Experimental data indicate that, when used concomitantly, ibuprofen may inhibit the effect of low doses of acetylsalicylic acid on platelet aggregation. However, limitations in extrapolating these data to the clinical situation do not allow definitive conclusions to be drawn that regular long-term use of ibuprofen may reduce the cardioprotective effect of low-dose acetylsalicylic acid. With unsystematic use of ibuprofen, such clinically significant effects are considered unlikely.
Ibuprofen should be used with caution in combination with the following medications.
Anticoagulants: NSAIDs may enhance the effect of anticoagulants such as warfarin.
Antihypertensives (ACE inhibitors and angiotensin II antagonists) and diuretics: NSAIDs may reduce the therapeutic effect of these drugs.
In some patients with impaired renal function (dehydrated patients or the elderly with impaired renal function), concomitant use of an ACE inhibitor or angiotensin II antagonist and drugs that inhibit COX may lead to a further deterioration of renal function, including possible acute renal failure, which is usually reversible. These interactions should be taken into account when the patient is simultaneously using a selective COX-2 inhibitor and ACE inhibitors or angiotensin II antagonists.
Therefore, such combinations should be prescribed with caution, especially in elderly patients. If long-term treatment is necessary, adequately hydrate the patient and consider monitoring renal function at the beginning of combination treatment, as well as at regular intervals thereafter. Diuretics may increase the risk of NSAID renal toxicity.
Corticosteroids: increased risk of ulcers and bleeding in the gastrointestinal tract.
Antiplatelet agents and selective serotonin reuptake inhibitors: the risk of gastrointestinal bleeding increases.
Cardiac glycosides: NSAIDs may enhance cardiac dysfunction, reduce glomerular filtration function of the kidneys and increase plasma levels of glycosides.
Lithium: There is evidence of a potential increase in plasma lithium levels.
Methotrexate: There is a potential for increased plasma levels of methotrexate.
Cyclosporine: increased risk of nephrotoxicity.
Mifepristone: NSAIDs should not be used earlier than 8 to 12 days after using mifepristone as they may reduce its effectiveness.
Tacrolimus: There may be an increased risk of nephrotoxicity when NSAIDs and tacrolimus are used concomitantly.
Zidovudine: An increased risk of hematological toxicity is known when zidovudine and NSAIDs are used in combination. There is evidence of an increased risk of developing hemarthrosis and hematoma in HIV-infected patients with hemophilia using concomitant treatment with zidovudine and ibuprofen.
Quinolone antibiotics: Concomitant use with NSAIDs may increase the risk of seizures.
Sulfonylureas and phenytoin: the effect of the drugs may be enhanced.
Overdose
Overdose
Use of the drug by children at a dose above 400 mg/kg body weight may lead to symptoms of intoxication. In adults, the dose effect is less pronounced. T½ in case of overdose is 1.5–3 hours.
Symptoms of intoxication. Nausea, vomiting, pain in the epigastric region, very rarely – diarrhea. Tinnitus, headache, and gastrointestinal bleeding may occur. With more severe poisoning, toxic damage to the central nervous system may occur, manifested in the form of vertigo, drowsiness, and sometimes agitation, disorientation or coma.
Sometimes patients experienced seizures. In severe poisoning, hyperkalemia and metabolic acidosis, an increase in prothrombin time/international normalized ratio (probably due to interaction with coagulation factors circulating in the bloodstream) may occur. AKI, liver damage, hypotension, respiratory depression, and cyanosis may occur. In patients with asthma, exacerbation of asthma is possible.
Treatment. Treatment should be symptomatic and supportive and include airway management and monitoring of cardiac function and vital signs until the patient’s condition returns to normal.
It is recommended that activated carbon be administered orally within 1 hour after administration of a potentially toxic dose of the drug. Frequent or prolonged muscle spasms should be treated with diazepam or lorazepam. In case of asthma, bronchodilators should be used.
Manufacturer
Manufacturer
Reckitt Benckiser Healthcare International Ltd, UK
Additional information
| Manufacturer | Reckitt Benckiser Healthcare International Ltd, United Kingdom |
|---|---|
| Medication form | pills |
| Brand | Reckitt Benckiser Healthcare International Ltd |
Other forms…
Related products
Buy Nurofen 12+, 200 mg 12 pcs with delivery to USA, UK, Europe and over 120 other countries.