No products in the cart.
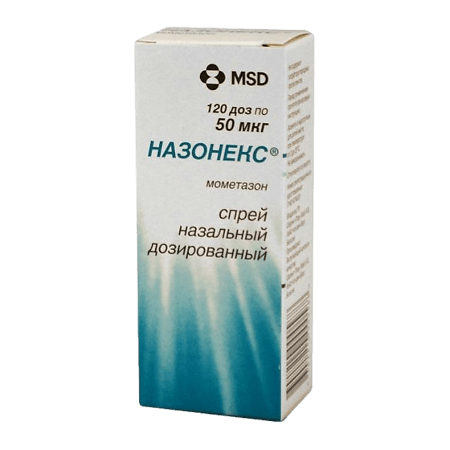
Nasonex, spray 50 mcg/dose 120 doses
€28.20 €24.44
Out of stock
(E-mail when Stock is available)
Description
Nasonex is an anti-allergic, anti-inflammatory.
Pharmacodynamics
Mometasone furoate is a synthetic glucocorticosteroid for topical use. It has anti-inflammatory and anti-allergic effects when used in doses at which no systemic effects occur. It inhibits release of inflammatory mediators, increases production of lipomodulin, which is an inhibitor of phospholipase A, which causes decrease in release of arachidonic acid and, correspondingly, inhibition of synthesis of products of arachidonic acid metabolism – cyclic endoperoxides, PG.
Prevents marginal accumulation of neutrophils, reduces inflammatory exudate and lymphokine production, inhibits macrophage migration, leads to a decrease in infiltration and granulation processes. It reduces inflammation due to decrease in formation of chemotaxis substance (effect on “late” allergic reactions) and inhibits development of “immediate” allergic reaction (conditioned by inhibition of production of arachidonic acid metabolites and decrease in release of inflammatory mediators from mast cells).
. In studies with provocation tests with application of antigens to the nasal mucosa high anti-inflammatory activity of Nasonex was demonstrated both in the early and late stages of the allergic reaction, which was confirmed by reduction (compared to placebo) of histamine and eosinophil activity as well as reduction (compared to baseline) of eosinophil, neutrophil and epithelial cell adhesion proteins.
Pharmacokinetics
Mometasone furoate has negligible bioavailability (≤0.1%) and is almost undetectable in plasma when administered by intranasal inhalation (even when using a sensitive method of determination with a sensitivity threshold of 50 pg/ml). Therefore, there are no relevant pharmacokinetic data for this dosage form. The suspension is very poorly absorbed from the gastrointestinal tract, so the small amount that may enter the gastrointestinal tract after inhalation into the nasal cavity is still undergoing active primary metabolism before excretion with urine or bile.
Indications
Indications
Treatment of allergic rhinitis (seasonal and year-round) in adults, adolescents and children over 2 years of age;
Exacerbation of sinusitis (complex therapy with antibiotics) in adults (including the elderly) and children over 12 years of age;
Prevention of moderate to severe seasonal allergic rhinitis (recommended 2–4 weeks before the expected start of the dusting season).
Pharmacological effect
Pharmacological effect
Nasonex – antiallergic, anti-inflammatory.
Pharmacodynamics
Mometasone furoate is a synthetic glucocorticosteroid for topical use. It has anti-inflammatory and antiallergic effects when used in doses at which systemic effects do not occur. Inhibits the release of inflammatory mediators, increases the production of lipomodulin, which is an inhibitor of phospholipase A, which causes a decrease in the release of arachidonic acid and, accordingly, inhibition of the synthesis of arachidonic acid metabolic products – cyclic endoperoxides, PGs.
Prevents the marginal accumulation of neutrophils, reduces inflammatory exudate and the production of lymphokines, inhibits the migration of macrophages, and leads to a reduction in the processes of infiltration and granulation. Reduces inflammation by reducing the formation of a chemotaxis substance (impact on “late” allergy reactions), inhibits the development of an “immediate” allergic reaction (due to inhibition of the production of arachidonic acid metabolites and a decrease in the release of inflammatory mediators from mast cells).
In studies with provocative tests when applying antigens to the nasal mucosa, the high anti-inflammatory activity of Nasonex was demonstrated in both the early and late stages of the allergic reaction, which was confirmed by a decrease (compared to placebo) in the level of histamine and eosinophil activity, as well as a decrease (compared to the baseline) in the number of eosinophils, neutrophils and epithelial cell adhesion proteins.
Pharmacokinetics
Mometasone furoate has negligible bioavailability (≤0.1%) and when administered by intranasal inhalation, it is practically undetectable in blood plasma (even when using a sensitive detection method with a sensitivity threshold of 50 pg/ml). Therefore, there are no relevant pharmacokinetic data for this dosage form. The suspension is very poorly absorbed from the gastrointestinal tract, so the small amount that can enter the gastrointestinal tract after inhalation into the nasal cavity undergoes active primary metabolism even before excretion with urine or bile.
Active ingredient
Active ingredient
Mometasone
Composition
Composition
Active ingredient:
Mometasone furoate (as monohydrate) 50 mcg;
Excipients:
Dispersed cellulose BP 65 cps;
Glycerol;
Sodium citrate dihydrate;
Citric acid monohydrate;
Polysorbate 80;
Benzalkonium chloride;
Phenylethyl alcohol;
Purified water.
Pregnancy
Pregnancy
After intranasal use of the drug at the maximum therapeutic dose, mometasone is not detected in the blood plasma even at the minimum concentration; therefore, fetal effects can be expected to be negligible and potential reproductive toxicity very low.
However, due to the fact that special, well-controlled studies of the drug’s effect in pregnant women have not been conducted, Nasonex should be prescribed to pregnant women, breastfeeding mothers, or women of childbearing age only if the expected benefit from its use justifies the potential risk to the fetus and newborn.
Newborns whose mothers used corticosteroids during pregnancy should be carefully examined to identify possible adrenal hypofunction.
Contraindications
Contraindications
Hypersensitivity to any component of the drug;
The presence of an untreated local infection involving the nasal mucosa;
Recent surgery or trauma to the nose (before the wound heals);
Tuberculosis infection (active or latent) of the respiratory tract, untreated fungal, bacterial, viral systemic infection or infection caused by Herpes simplex with eye damage (as an exception, the drug can be prescribed in these cases as directed by a doctor with great caution);
Children under 2 years of age (no data on safety of use).
Side Effects
Side Effects
For the treatment of seasonal or year-round allergic rhinitis.
In adults:
nosebleeds (overt nosebleeds or blood-stained mucus or blood clots)
pharyngitis,
burning sensation in the nose,
irritation of the nasal mucosa.
Nosebleeds, as a rule, stopped on their own and were not severe; they occurred at a rate slightly higher than with placebo (5%), but equal to or less than with other intranasal corticosteroids that were used as active controls (in some of them the incidence of nosebleeds was up to 15%). The incidence of all other adverse events was comparable to that observed with placebo.
In children:
nosebleeds,
headache,
feeling of irritation in the nose,
sneezing.
The incidence of these adverse events in children was comparable to the incidence when using placebo.
In the treatment of exacerbations of sinusitis (when using Nasonex spray as an adjuvant).
In adults and adolescents:
headache,
pharyngitis,
burning sensation in the nose,
irritation of the nasal mucosa.
Nosebleeds were moderate, and their incidence with Nasonex was also comparable to the incidence of nosebleeds with placebo (5% versus 4%, respectively).
Very rarely, with intranasal use of GCS, cases of perforation of the nasal septum or increased intraocular pressure have been reported.
Interaction
Interaction
Combination therapy with loratadine was well tolerated by patients. Interaction studies with other drugs have not been conducted.
Overdose
Overdose
Symptoms: with long-term use of GCS in high doses, as well as with the simultaneous use of several GCS, inhibition of the function of the hypothalamic-pituitary-adrenal system is possible.
Treatment: due to the low (less than 0.1%) systemic bioavailability, it is unlikely that in case of accidental or intentional overdose, any measures will be required other than monitoring the patient and then continuing treatment at the recommended dose.
Storage conditions
Storage conditions
At a temperature of 2–25 °C.
Shelf life
Shelf life
2 years.
Manufacturer
Manufacturer
Organon Heist bv, Belgium
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | At a temperature of 2-25 °C. |
| Manufacturer | Schering-Plough Labo N.V., Belgium |
| Medication form | dosed nasal spray |
| Brand | Schering-Plough Labo N.V. |
Related products
Buy Nasonex, spray 50 mcg/dose 120 doses with delivery to USA, UK, Europe and over 120 other countries.