No products in the cart.
Mycophenolate-Teva, capsules 250 mg 100 pcs
€64.75 €56.11
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group:immunosuppressive
ATX code: L04AA06
Pharmacological properties
Pharmacodynamics
An immunosuppressant, inosine monophosphate dehydrogenase inhibitor Mycophenolate mofetil (MMF) is the 2-morpholinoethyl ester of mycophenolic acid (MPA).
MFC is a potent, selective noncompetitive and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), which inhibits the synthesis of guanosine nucleotides de novo. The mechanism by which MFC suppresses the enzymatic activity of IMPDH appears to be related to the fact that MFC structurally mimics both the nicotinamide dinucleotide phosphate cofactor and the catalyzing water molecule. This prevents the oxidation of IMF into xanthose-5-monophosphate, a crucial step in guanosine nucleotide biosynthesis de novo. MFC has a more pronounced cytostatic effect on lymphocytes than on other cells because the proliferation of T- and B-lymphocytes is highly dependent on purine synthesis de novo, while other cell types can switch to bypass metabolic pathways.
Effectiveness
In clinical trials to prevent rejection after kidney, heart and liver transplants, mycophenolate mofetil was used in combination with the following drugs: Antithymocyte immunoglobulin, Orthoclon OCT-Z (murine monoclonal antibodies), cyclosporine, and glucocorticosteroids.
Prevention of graft rejection
Adults
. The safety and efficacy of MMF in combination with glucocorticosteroids and cyclosporine were evaluated in patients after kidney, heart, and liver transplantation.
Children
The safety, pharmacokinetics and efficacy of MMF in combination with glucocorticosteroids and cyclosporine in children after kidney transplantation were evaluated in a study involving 100 children aged 3 months to 18 years.
Kidney transplantation
Adults
. In combination with glucocorticosteroids and cyclosporine, MMF statistically significantly reduces the incidence of therapy failure in the first 6 months after transplantation and histologically proven rejection during therapy; at a dose of 2 g/day it reduces the cumulative rate of graft death and mortality at 12 months after renal transplantation, but at a dose of 3 g/day it increases the rate of premature study dropout for any reason.
Children
In children after kidney transplantation in all age groups, MMF (powder for suspension preparation) was administered at doses of 600 mg/m2 twice daily (up to 1 g twice daily).
The overall incidence of histologically proven rejection by 6 months post-transplantation was comparable to that in adults and was similar across age groups. The cumulative rates of graft death (5%) and mortality (2%) at 12 months post-transplant were comparable to those observed in adult kidney transplant recipients.
Cardiac transplantation
Return
. There were no differences in the frequency of histologically proven rejection resulting in impaired hemodynamics in the MMF and azathioprine groups.
Survival rates
In terms of mortality and repeat transplants in heart transplantation, MMF was superior to azathioprine.
Liver transplantation
. MMF in combination with glucocorticosteroids and cyclosporine was more effective than azathioprine in preventing acute rejection and provided the same survival rate as azathioprine.
Doclinical safety data
. At doses 2 to 3 times therapeutic in kidney transplant patients and 1.3 to 2 times therapeutic in heart transplant patients, MMF did not stimulate tumor formation and did not affect the fertility of male rats.
Two genotoxicity tests indicated that at doses with serious toxic effects, MMF has the potential to cause chromosomal instability. Other genotoxicity tests showed no mutagenic activity in the drug.
In fertility and reproductive experiments in female rats, oral administration of the drug caused malformations (including anophthalmia, agnathia and hydrocephaly) in the first generation of offspring without any toxic effect on the mother. No effect on fertility and reproductive performance was observed in subsequent offspring generations.
In teratogenicity studies in rats, fetal resorption and birth defects in offspring (including anophthalmia, agnathia and hydrocephaly in rats and cardiovascular, renal, heart and kidney malformations, diaphragmatic and umbilical hernia in rabbit offspring) without evidence of toxic effects on the mother were observed.
In animal toxicology studies of MMF, the major lesions were localized to the hematopoietic and lymphoid organs and occurred at levels of systemic exposure to the drug that were equivalent to or less than the exposure levels at the clinical dose of 2 g per day recommended for patients after kidney transplantation. The nonclinical toxicity profile of MMF is consistent with adverse events reported in human clinical trials that have yielded safety data more relevant to the patient population (see “Adverse Effects”).
Pharmacokinetics
The pharmacokinetic characteristics of MMF have been studied in kidney, heart and liver transplant patients. In general, the pharmacokinetic profile of MMF is the same in patients after kidney and heart transplantation. In the early post-transplant period, liver transplant patients receiving MMF at a dose of 1.5 g have the same concentrations of MMF as kidney transplant patients receiving MMF at a dose of 1 g.
Intake
After oral administration, rapid and complete absorption and complete presystemic metabolism of MMF occurs to form the active metabolite, MFC. Bioavailability of MMF when administered orally, according to the area under the curve “concentration-time” (AUCMFC), is, on average, 94% of that when administered intravenously. After oral administration, plasma concentrations of MMF are undetectable (below the threshold of determination – 0.4 µg/ml).
In the early post-transplant period (up to 40 days after kidney, heart, or liver transplantation), mean AUCMFC values were approximately 30% lower and maximum concentrations approximately 40% lower than in the late post-transplant period (3-6 months after transplantation). Food intake had no effect on the degree of MMF absorption (AUCMFC) when administered at 1.5 g twice daily in patients after kidney transplantation. However, the maximum concentration of MFC is decreased by 40% when the drug is taken with meals.
Distribution
In general, approximately 6-12 hours after drug administration, a secondary increase in plasma concentrations of MFC is observed, indicating hepatic-intestinal recirculation of the drug. When coadministration of colestyramine, the AUCMFC is decreased by approximately 40%, indicating interruption of hepatic-intestinal recirculation.
In clinically relevant concentrations, MFC is 97% bound to plasma albumin.
Metabolism
MPC is metabolized primarily by glucuronyltransferase (UGT1A9 gene isoform) to form the pharmacologically inactive phenolic glucuronide MFC (MFCG). In vivo MFKG is converted back to free MFKG during hepatic-intestinal recirculation to form acylglucuronide, which has pharmacological activity and may cause some side effects of MFKG (diarrhea, leukopenia).
Elimation
After oral administration of radioactively labeled MMF, 93% of the dose received is excreted in the urine and 6% in the feces. Most (about 87%) of the administered dose is excreted in the urine as MFKG. Minor amounts of the drug (<1% of the dose) are excreted with the urine as MFCC.
Clinically detectable concentrations of MFCs and MFCG are not eliminated by hemodialysis. However, at higher concentrations of MFKH (>100 µg/ml), some of it can be removed. Bile acid sequestrants like colestiramine decrease the AUCMFC by interrupting hepatic-intestinal recirculation.
The distribution of MFC depends on several transporters: the transport polypeptide of organic anions (TPOA) and multidrug-resistance-associated protein-2 (BAMLU-2). TPOA isoforms, BAMLU-2, and breast cancer resistance protein (BRPM) are transporters associated with glucuronide excretion through the bile. Multiple drug resistance protein-1 may also be involved in IFC transport, but its involvement is limited to the absorption process. MFC and its metabolites can potentially react with organic anion transporters in the kidneys.
Pharmacokinetics in special clinical cases
In a study with single drug administration in patients with severe chronic renal impairment (glomerular filtration rate <25 mL/min/1.73 m2 ) the AUCMFC was 28-75% greater than in healthy volunteers and patients with less severe renal impairment. After a single dose, the AUCMFC was 3-6 times greater in patients with severe renal impairment than in healthy volunteers and patients with moderate renal impairment, which is consistent with data on renal excretion of MFKG. There have been no studies on multiple administrations of MMF in severe chronic renal failure.
In patients with delayed renal graft function after transplantation, the mean AUC0-12 for MMF is comparable to that of patients in whom the graft began to function after transplantation without delay. Transient increases in free fraction and plasma concentrations of MFC may be observed in patients with delayed renal graft function. It is probably not necessary to adjust the MMF dose in these patients (see section “Dosing in special cases”). The mean AUC0-12 for MFKH in plasma was 2-3 times greater than in patients in whom the graft began to function after transplantation without delay.
In patients with a primary nonfunctioning graft after kidney transplantation, there was an increase in plasma concentrations of MFKG; cumulation of MFKG, if any, was observed to a much lesser extent compared with MFKG.
Patients with liver damage. Volunteers with alcoholic cirrhosis showed no changes in the pharmacokinetics of MFC and MFCG after oral administration of MMF. The effect of hepatic pathology on this process probably depends on the specific disease. In the case of liver disease with predominant biliary tract involvement (e.g., primary biliary cirrhosis), changes in the pharmacokinetics of MFC and MFCG cannot be excluded.
In pediatric patients (≤18 years of age) who have undergone a kidney transplant, after oral administration of MMF at a dose of 600 mg/m2 twice daily (up to a maximum of 1 g twice daily), the AUC for MMF is comparable to that of adult kidney transplant patients receiving a dose of 1 g twice daily in the early and late post-transplant period. AUC values for MFC did not differ between age groups in the early and late post-transplant period.
In elderly and elderly patients (≥65 years), pharmacokinetics have not been studied.
Indications
Indications
The drug is used as combination therapy with cyclosporine and corticosteroids.
Adults and children with a body surface area >1.25 m2 (approximate childhood age over 12 years):
Adults:
Active ingredient
Active ingredient
Composition
Composition
How to take, the dosage
How to take, the dosage
Ingestion.
Adults
Prevention of kidney transplant rejection./em>
The drug should be started within 72 hours of transplant surgery. For patients with renal transplants it is recommended to take 1 g twice a day (daily dose 2 g). Although a dose of 1.5 g twice daily (daily dose of 3 g) has also been shown in clinical trials to be safe and effective, its benefits in terms of efficacy in patients after kidney transplantation have not been established. Patients who received 2 g of MMF per day had an overall better safety profile than those who received a daily dose of 3 g.
Prevention of heart transplant rejection
The drug should be started within 5 days after transplant surgery. Recommended dosing regimen is 1.5 g 2 times daily (daily dose 3 g).
Prevention of liver transplant rejection
The drug should be started as soon as possible after transplant surgery (depending on the patient’s ability to tolerate the drug). The recommended dosing regimen is 1.5 g 2 times daily (3 g daily dose).
Dosing in special cases
In case of neutropenia (absolute neutrophil count <1300 in 1 µl of blood) it is necessary to interrupt treatment with MMF or reduce its dose and carefully monitor the patient (see “Special Precautions. See section “Special Precautions”).
In patients with severe chronic renal insufficiency (glomerular filtration rate less than 25 mL/min/1.73 m2) outside the immediate post-transplant period, doses above 1 g 2 times daily should be avoided. No data are available for patients with severe renal failure who have undergone heart or liver transplantation (see section “Special Precautions”).
Dose adjustment is not required in patients with delayed renal transplant function (see section “Pharmacokinetics in Special Clinical Cases”).
Patients who have undergone renal transplantation and have severe liver parenchymal damage do not require dose adjustments (see section “Pharmacokinetics in Special Clinical Cases”). No data are available for patients with severe liver parenchyma damage who have undergone heart transplantation.
In older and elderly patients (â¥65 years) who have undergone a kidney transplant, the recommended dose is 1 g 2 times daily and 1.5 g 2 times daily after heart or liver transplantation (see section “Special Precautions”).
children:
Interaction
Interaction
Aciclovir
When MMF and acyclovir were used concomitantly, higher plasma concentrations of MFCG and acyclovir were observed than when each drug was used separately. Since plasma concentrations of MMFH, as well as acyclovir, are elevated in renal insufficiency, there is a possibility that the two drugs compete with respect to tubular secretion, which may lead to further increases in concentrations of both drugs.
Antacids and proton pump inhibitors (PPIs)
. When MMF was coadministered with antacids (aluminum and magnesium hydroxide) and with proton pump inhibitors (lansoprazole and pantoprazole), a decrease in MMF concentrations was observed. However, there was no significant difference between the rates of graft rejection in patients taking MMF concomitantly with and without IPN drugs. This conclusion theoretically extends to antacids as well, because when they are taken concomitantly with MMF, MFC concentrations are reduced to a much lesser extent than when MMF is taken concomitantly with IPNs.
Colestyramine
. A 40% reduction in AUCMFC was observed after a single dose of MMF of 1.5 g in healthy volunteers who had previously taken 4 g of colestiramine 3 times daily for 4 days. Caution should be exercised when concomitant use of MMF and drugs affecting hepatic-intestinal recirculation (see section “Cautions”).
Cyclosporine
MMF does not affect the pharmacokinetics of cyclosporine. However, cyclosporine does affect hepatic-intestinal recirculation of MMF, which may result in an increase in AUCMFC of approximately 30% when discontinuing cyclosporine in patients after renal transplantation receiving MMF and cyclosporine (compared to patients receiving sirolimus or belatacept with similar doses of MMF). In contrast, when patients switch from cyclosporine therapy to therapy with immunosuppressants that do not affect hepatic-intestinal recirculation of MMF, changes in MMF exposure should be expected.
Telmisartan
Concomitant use with MMF results in a decrease in MFC concentrations of approximately 30%. Telmisartan influences the excretion of MMF by increasing the expression of peroxisome proliferator-activated receptor gamma, which in turn increases the expression and activity of the UGT1A9 gene. No clinical manifestations of drug interactions were found when comparing graft rejection rates and adverse event profiles in patients receiving MMF with or without concomitant telmisartan therapy.
Ganciclovir
Based on a study with single oral administration of the recommended doses of MMF and intravenous administration of ganciclovir, given the known effects of renal impairment on MMF pharmacokinetics (see and ganciclovir) and ganciclovir, it can be assumed that the simultaneous use of these two drugs (competing in the process of tubular secretion) will lead to increased concentrations of MMF and ganciclovir. No significant change in the pharmacokinetics of MMF is expected, so there is no need to adjust the dose of MMF. If MMF and ganciclovir (or its prodrugs such as valganciclovir) are used in patients with renal impairment, patients should be monitored closely.
Peroral contraceptives
. In a study involving 18 women with psoriasis, concomitant administration for 3 menstrual cycles of MMF (1 g twice daily) with combined oral contraceptives containing ethinylestradiol (0.02-004 mg) and levonorgestrel (0.05-0.2 mg), desogestrel (0.15 mg), or gestoden (0.05-0.1 mg), no clinically significant effects of MMF on progesterone, luteinizing hormone (LH) and follicle stimulating hormone (FSH) concentrations were found. Thus, MMF had no effect on the suppression of ovulation by oral contraceptives. The pharmacokinetics of oral contraceptives did not change to a clinically significant degree when co-administered with MMF.
Special Instructions
Special Instructions
Malignancies
As with combined immunosuppression in general, and with MMF as a component of an immunosuppressive regimen, there is an increased risk of lymphoma and other malignancies, especially of the skin (see section “Adverse effects”). This risk appears to be related not to the use of any drug per se, but to the intensity and duration of immunosuppression.
As with all patients at increased risk for skin cancer, time exposure to sun and UV rays should be limited by wearing appropriately covered clothing and using sunscreens with a high protective factor.
Infections
Excessive suppression of the immune system may also increase susceptibility to infections, including opportunistic, sepsis, and other fatal infections (see side effects). Such cases include reactivation of latent viral infections such as hepatitis B or C or infections caused by polyomaviruses. Cases of hepatitis due to reactivation of hepatitis B or C viruses have been reported in hepatitis B or C carrier patients receiving immunosuppressive therapy. Cases of PML associated with JC virus, sometimes fatal, have been observed in patients receiving MMF. These cases have been reported to have additional risk factors for PML, including immunosuppressive therapy and immune deterioration. In immunosuppressed patients with neurologic symptoms, a differential diagnosis of PML should be made and a consultation with a neurologist should be recommended.
In kidney transplant patients who have received MMF, there have been cases of BK virus-associated nephropathy (BK virus – associated nephropathy). This infection can have serious consequences, sometimes leading to the death of the kidney transplant. Patients receiving MMF should be monitored to identify those at risk for BK virus-associated nephropathy. If symptoms of BK-virus-associated nephropathy develop, reduction of immunosuppression should be considered.
Blood and immune system
Cases of PKKA have been observed in patients taking MMF in combination with other immunosuppressive drugs. The mechanism of PKCA with MMF is unknown, as is the contribution of other immunosuppressive drugs and their combinations. In some cases, PKKA has been reversible after MMF drug dosage reduction or withdrawal. However, in transplant patients, decreased immunosuppression may compromise the graft.
Patients receiving MMF should be informed to report any signs of infection, bleeding, hemorrhage or other signs of bone marrow suppression to their physician immediately.
In treatment with MMF, detailed blood counts should be determined weekly during the first month, twice monthly during the second and third months of treatment, and monthly thereafter for the first year. Particular attention should be paid to the possibility of neutropenia. Neutropenia may be associated with MMF administration as well as with the use of other drugs, viral infections or a combination of these causes (see section “Dosing in special cases”). If neutropenia (absolute neutrophil count <1.3Ã103/μL) occurs, MMF treatment should be discontinued or the dose should be reduced, with close monitoring of these patients.
In the course of MMF treatment, vaccination may be less effective; live, attenuated vaccines should be avoided (see section “Interactions with other medications”). Anti-influenza vaccination can be given according to national guidelines.
Gastrointestinal tract
The administration of MMF may be accompanied by adverse gastrointestinal reactions (gastrointestinal mucosal ulceration, gastrointestinal bleeding, perforations of the GI tract). Caution should be exercised when using MMF in patients with diseases of the digestive tract in the acute stage.
MMP is an IMFDH inhibitor, so theoretically, it should not be used in patients with rare genetically determined hereditary hypoxanthine anguanine phosphoribosyltransferase deficiency (Lesch-Nayen and Kelly-Zygmiller syndromes).
Interactions
. Caution should be exercised when switching from combination therapy involving immunosuppressants that have an effect on hepatic-intestinal recirculation of IFCs (e.g., cyclosporine) to therapy with drugs devoid of this effect (e.g., sirolimus and belatacept) and vice versa. This transition may lead to a change in the exposure of MFCs. Caution should be exercised when concomitant use of drugs that affect the hepatic-intestinal cycle of MMF (e.g., colestyramine, antibiotics), due to their ability to decrease the plasma concentration and efficacy of MMF.
The risk/benefit ratio of concomitant use of MMF and tacrolimus or sirolimus has not been established. MMF is not recommended for concomitant use with azathioprine because both drugs inhibit bone marrow function, and their concomitant use has not been studied.
Special patient groups
The drug Mycophenolate-Teva is contraindicated during pregnancy and during breast-feeding. Doses greater than 1 g 2 times daily should be avoided in patients with severe CKD (see Sections “Pharmacokinetics in Special Clinical Cases” and “Dosing in Special Cases”).
Dose adjustment is not necessary in patients with delayed renal transplant function, but they should be closely monitored (see sections “Pharmacokinetics in Special Clinical Cases” and “Dosing in Special Cases”). No data are available for heart or liver transplant patients with severe renal insufficiency.
The risk of adverse events may be higher in elderly patients than in younger patients (see section “Adverse effects”).
Management of the drug
Because MMF has shown teratogenic effects in experiments in rats and rabbits, the capsules of Mycophenolate-Teva should not be broken. It is necessary to avoid inhalation of powder contained in Mycophenolate-Teva capsules or its contact with skin or mucous membranes. If this happens, you should rinse the area thoroughly with soap and water, and the eyes with just water.
Influence on driving and operating machinery
When using the drug Mycophenolate-Teva caution should be exercised while driving vehicles and performing potentially dangerous activities requiring increased concentration and rapid psychomotor reaction, as dizziness may occur.
Synopsis
Synopsis
Contraindications
Contraindications
Side effects
Side effects
The profile of adverse events associated with immunosuppressant use is often difficult to establish because of the underlying disease and the concurrent use of many other medications.
Data from clinical trials
. The main adverse events associated with the use of MMF in combination with cyclosporine and glucocorticosteroids to prevent renal, cardiac or hepatic transplant rejection are diarrhea, leukopenia, sepsis and vomiting; There is also evidence of an increased incidence of certain types of infections, such as opportunistic infections (see See section “Special Indications”).
Malignant neoplasms
As with combined immunosuppression in general, and with MMF as a component of an immunosuppressive regimen, there is an increased risk of lymphoma and other malignancies, especially of the skin (see section “Adverse effects”). In controlled clinical trials in patients who underwent kidney, heart, or liver transplantation and were followed for at least 1 year, lymphoproliferative disease or lymphoma developed in 0.4-1% of patients who received MMF (in doses of 2 or 3 g daily) in combination with other immunosuppressive agents. Skin cancer (excluding melanoma) occurred in 1.6-3.2% of patients, malignant neoplasms of other types in 0.7-2.1% of patients. Three-year safety data in patients after kidney or heart transplantation showed no unexpected changes in the incidence of malignant neoplasms compared with one-year rates. After liver transplantation, patients were followed for at least 1 year but less than 3 years.
Opportunistic infections
The risk of opportunistic infections is increased in all post-transplant patients and increases with the degree of immunosuppression (see section “Special Instructions”). In controlled clinical trials with MMF (2 or 3 g daily) in combination with other immunosuppressants in patients followed for 1 year after kidney transplantation (at a dose of 2 g daily), heart and liver, the most frequent infections were candidiasis of skin and mucous membranes, cytomegalovirus (CMV) infection: CMV viremia/CMV syndrome (13.5%) and infection caused by herpes simplex virus.
After kidney transplantation, sepsis (usually associated with CMV) occurred more frequently in patients receiving MMF than in patients in the control group. Patients who received MMF at a dose of 3 g had a higher incidence of sepsis than patients who received MMF at a dose of 2 g.
Children (3 months to 18 years)
The type of adverse reactions and incidence in a clinical trial with oral administration of 600 mg/m2 MMF 2 times daily in children aged 3 months to 18 years (N=100) were virtually indistinguishable from those in adult patients receiving the drug at a dose of 1 g 2 times daily. However, adverse reactions such as diarrhea, leukopenia, sepsis, infections, and anemia occurred more frequently (â¥10%) in children, especially those under 6 years of age.
In elderly and elderly patients (â¥65 years), when treated with MMF as part of combined immunosuppressive therapy, the risk of certain infections (including tissue invasive forms of manifest cytomegalovirus infection) and possibly gastrointestinal bleeding and pulmonary edema is higher than in younger patients (see section “Special indications”).
Adverse events noted in â¥10% and in 3-10% of patients receiving MMF in combination with cyclosporine and glucocorticosteroids in controlled studies of renal transplant rejection prevention (3 studies, 2 and 3 mg daily dose data), a controlled heart transplant study and a controlled liver transplant study.
adverse events after kidney transplantation (n=991)*
Adverse events after heart transplantation (n=289)**
/td>
Adverse events after liver transplantation (n=277)***
Infectious and parasitic diseases
3 – < 10%
infections, sepsis
infections, sepsis
infections, sepsis
Blood and lymphatic disorders system
â¥10%
anemia (including hypochromic), leukocytosis, leukopenia, thrombocytopenia
anemia (including hypochromic), leukocytosis, leukopenia, thrombocytopenia, ecchymosis
anemia (including hypochromic), leukocytosis, leukopenia, thrombocytopenia
3 – < 10%
ecchymoses, polycythemia, bleeding
petechiae, increased prothrombin and thromboplastin time, bleeding
ecchymoses, increased prothrombin time, pancytopenia, bleeding
Endocrine system disorders
3 – < 10%
diabetes mellitus, parathyroid gland disease (elevated parathyroid hormone levels)
diabetes mellitus, Cushing’s syndrome, hypothyroidism
/p>
diabetes mellitus
disorders of metabolism and nutrition
â¥10%
acidosis (metabolic or respiratory), hypervolemia, weight gain
3 – < 10%
acidosis (metabolic or respiratory), dehydration, hypervolemia, weight gain
alkalosis, dehydration, gout, hypovolemia, hypoxia, respiratory acidosis, thirst, weight loss, hypervolemia
acidosis (metabolic or respiratory), dehydration, hypervolemia, hypoxia, hypovolemia, weight gain, weight loss
Nervous system disorders
â¥10%
/td>
dizziness, insomnia, tremor, headache
agitation, anxiety, confusion, depression, dizziness, hypertonicity, insomnia, paresthesias, drowsiness, tremor, agitation, headache
anxiety, confusion, depression, dizziness, insomnia, paresthesia, tremor, headache
/p>
3 – < 10%
anxiety, depression, hypertonicity, paresthesias, drowsiness
seizures, emotional lability, hallucinations, neuropathy, thought disorders, vertigo, dizziness
agitation, seizures, delirium, dry mouth, hypertonicity, hypoesthesia, neuropathy, psychosis, drowsiness, thought disorders, delirium, agitation
Visual disturbances
â¥10%
amblyopia
3 – < 10%
amblyopia, cataracts, conjunctivitis
visual disturbances, conjunctivitis, bleeding in the eye
/p>
visual disturbances, amblyopia, conjunctivitis
Hearing disturbances.Hearing and labyrinth disorders
3 – < 10%
deafness, ear pain, tinnitus
deafness
Cardiac disorders
/td>
â¥10%
arrhythmia, bradycardia, heart failure, pericardial effusion
tachycardia
3 – < 10%
angina, atrial fibrillation, tachycardia, palpitations
angina pectoris, arrhythmias (supraventricular and ventricular extrasystoles, atrial flutter and fibrillation, supraventricular and ventricular tachycardia), cardiac arrest, congestive heart failure, syncope
atrial fibrillation, arrhythmias, bradycardia, syncope
high blood pressure
low and high blood pressure
low and high blood pressure
3 – < 10%
low blood pressure, orthostatic hypotension, thrombosis, vasodilation
orthostatic hypotension, pulmonary hypertension, vasospasm, increased venous pressure
arterial thrombosis, vasodilation
disorders of the respiratory system, thoracic and mediastinal organs
â¥10%
increased cough, shortness of breath, pharyngitis, pneumonia, bronchitis
asthma, increased cough, shortness of breath, pharyngitis, pleural effusion, pneumonia, rhinitis, sinusitis
increased cough, dyspnea, pharyngitis, pneumonia, pleural effusion, sinusitis, atelectasis
3 – < 10%
asthma, pleural effusion, pulmonary edema, rhinitis, sinusitis
apnea, atelectasis, bronchitis, nasal bleeding, hemoptysis, hiccups, neoplasms, pneumothorax, pulmonary edema, increased sputum discharge, voice changes
asthma, bronchitis, nasal bleeding, hyperventilation, pneumothorax, pulmonary edema, airway candidiasis, rhinitis
.Gastrointestinal disorders
â¥10%
constipation, diarrhea, dyspepsia, nausea and vomiting, oral candidiasis, abdominal pain
constipation, diarrhea, dyspepsia, flatulence, nausea and vomiting, oral candidiasis, abdominal pain
/p>
anorexia, constipation, diarrhea, dyspepsia, flatulence, nausea and vomiting, mucosal candidiasis, hernia, peritonitis, ascites, abdominal pain, abdominal bloating
/p>
3 – < 10%
Anorexia, flatulence, gastroenteritis, gastrointestinal bleeding, gastrointestinal candidiasis, gingivitis, gum hyperplasia, intestinal obstruction, stomatitis, esophagitis, loss of appetite, gastritis, hernia, bloating
anorexia, dysphagia, gastroenteritis, gingivitis, gum hyperplasia, melena, stomatitis, esophagitis, loss of appetite, hernia, bloating
dysphagia, gastritis, gastrointestinal bleeding, intestinal obstruction, melena, oral mucosal ulceration, esophagitis, rectal lesions, peptic ulcer
/p>
Liver and biliary tract disorders
/em>
â¥10%
increased lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT) activity, bilirubinemia
bilirubinemia, cholangitis, cholestatic jaundice, hepatitis, increased activity of liver enzymes (including aspartate aminotransferase (AST), alanine aminotransferase (ALT))
3 – < 10%
liver dysfunction, increased concentration of alkaline phosphatase, hepatitis, increased activity of liver enzymes (including aspartate aminotransferase (AST), alanine aminotransferase (ALT))
Hepatic dysfunction, increased alkaline phosphatase concentration, jaundice, increased liver enzyme activity (including aspartate aminotransferase (AST), alanine aminotransferase (ALT))
jaundice
dermatological and subcutaneous tissue disorders
dermatological and subcutaneous tissue disorders/p>
â¥10%
acne, herpes simplex
/p>
acne, herpes simplex, shingles, rash
/p>
rash, itching, increased sweating, wounds that are hard to heal
/p>
3 – < 10%
Hair loss, benign skin neoplasms, fungal dermatitis, shingles, hirsutism, itching, skin cancer, skin hypertrophy (including.Ñ. actinic keratoses), excessive sweating, skin ulcers, rashes
benign skin growths, fungal dermatitis, hemorrhages, itching, skin cancer, skin hypertrophy, increased sweating, skin ulcers, hard-to-heal wounds, cellulitis
acne, fungal dermatitis, hemorrhages, herpes simplex, herpes zoster, hirsutism, benign skin growths, skin ulcers, vesicular bullous rash, cellulitis, scrotal edema, abscesses/p>
Musculoskeletal and connective tissue disorders
â¥10%
/td>
back pain
leg cramps, muscle pain, muscle weakness, back pain
back pain
/p>
3 – < 10%
joint pain, leg cramps, muscle pain, muscle weakness
joint pain, neck pain
joint pain, leg cramps, muscle pain, muscle weakness, osteoporosis
Kidney and urinary tract disorders
â¥10%
hematuria, renal tubule necrosis, urinary tract infections
renal dysfunction (decreased renal function, increased concentration of serum creatinine), oliguria, urinary tract infections
renal dysfunction (decreased renal function, increased concentration of serum creatinine), oliguria, urinary tract infections
3 – < 10%
albuminuria, dysuria, hydronephrosis, pyelonephritis, frequent urination
dysuria, hematuria, nycturia, renal failure, frequent urination, incontinence and urinary retention/p>
acute renal failure, dysuria, hematuria, renal failure, frequent urination, incontinence
disorders of the genitals, mammary glands
3 – < 10%
impotence
impotence
General disorders and disorders at the site of administration
â¥10%
asthenia, fever, infections, chest pain, edema, sepsis
asthenia, fever, chills, infections, chest pain, edema, sepsis
asthenia, fever, chills, infections, chest pain, edema, sepsis
3 – < 10%
cysts (including lymphocele and hydrocele), facial edema, flu-like syndrome, malaise, pelvic pain
cysts (including lymphocele and hydrocele), facial edema, flu-like syndrome, malaise, pelvic pain, pale skin
cysts (including lymphocele and hydrocele), flu-like syndrome, malaise, neck pain
.Laboratory and instrumental data
â¥10%
hypercholesterolemia, hyperglycemia, hyperkalemia, hypokalemia, hypophosphatemia
hyperbilirubinemia, increased residual nitrogen, increased concentration of creatinine, increased serum lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT) enzyme activity, hypercholesterolemia, hyperglycemia, hyperkalemia, hyperlipidemia, hyperuricemia, hypokalemia, hypomagnesemia, hyponatremia
Hypo/p>
hyperbilirubinemia, increased residual nitrogen, increased concentration of creatinine, hyperglycemia, hyperkalemia, hypokalemia, hypocalcemia, hypoglycemia, hypomagnesemia, hypophosphatemia, hypoproteinemia
Hypoproteinemia/p>
3 – < 10%
increased alkaline phosphatase activity, increased enzyme activity (gammaglutamyl transpeptidase, lactate dehydrogenase (LDH), aspartate aminotransferase (AST) alanine aminotransferase (ALT)) in serum, increased concentration of serum creatinine, hypercalcemia, hyperlipidemia, hypocalcemia, hypoglycemia, hypoproteinemia, hyperuricemia/p>
increased alkaline phosphatase activity, hypocalcemia, hypochloremia, hypoglycemia, hypoproteinemia, hypophosphatemia
increase in the activity of alkaline phosphatase, increased enzyme activity (aspartate aminotransferase (AST), alanine aminotransferase (ALT)) in serum, hypercholesterolemia, hyperlipidemia, hyperphosphatemia, hyponatremia
Hypophosphatemia in blood/p>
*(total n=1483), **(total n=578), ***(total n=564)
In three controlled studies on the prevention of renal transplant rejection, the safety profile of MMF at a daily dose of 2 g was slightly better than that at a daily dose of 3 g.Post-registration use of the drug
Infections: isolated cases of severe, life-threatening infections (meningitis, infective endocarditis), increased incidence of some infections such as tuberculosis and atypical mycobacterial infections.
Cases of progressive multifocal leukoencephalopathy (PML), sometimes fatal, have been observed in patients taking MMF. These cases have been reported to have additional risk factors for PML, including immunosuppressive therapy and immune deterioration.
There have been cases of BK virus-associated nephropathy (BK virus – associated nephropathy) in patients taking MMF. This infection can lead to serious consequences, sometimes death of the kidney transplant.
Blood and immune system disorders:Cases of partial red cell aplasia (PKCA) and hypogammaglobulinemia have been observed in patients taking MMF in combination with other immunosuppressive drugs.
Developmental abnormalities:In the post-registration period, cases of congenital malformations have been reported in children of patients who took MMF during pregnancy in combination with other immunosuppressive drugs.
Pregnancy, postpartum and perinatal conditions:Cases of spontaneous miscarriage have been reported in patients receiving mycophenolate mofetil, mainly in the first trimester of pregnancy.
Digestive system disorders:colitis (sometimes of cytomegalovirus etiology), pancreatitis, isolated cases of atrophy of intestinal villi.
The other adverse reactions observed during post-registration use of the drug are not different from the adverse reactions reported in controlled clinical trials.
Overdose
Overdose
Data on MMF overdose have been reported in clinical studies and in postmarketing use. The adverse events that developed in overdose were consistent with the known safety profile of MMF.
Overdose of MMF can be expected to be accompanied by excessive immunosuppression, increased susceptibility to infection, and inhibition of bone marrow function.
Treatment:If neutropenia develops, MMF administration should be discontinued or the dose should be reduced. MMF cannot be removed from the body by hemodialysis. However, at high plasma concentrations of MMFH (more than 100 µg/ml), small amounts are still eliminated. Drugs that bind bile acids, such as colestyramine, can help eliminate MFKH from the body by increasing its excretion.
Pregnancy use
Pregnancy use
The use of the drug Mycophenolate-Teva is contraindicated during pregnancy and in women of childbearing potential who do not use highly effective methods of contraception.
Before starting therapy, patients of childbearing potential, both men and women, should be informed about the increased risk of fetal death and congenital malformations; advice should be given on measures to prevent pregnancy and its planning.
Before initiating therapy with Mycophenolate-Teva, patients of childbearing potential should have a negative result of two pregnancy tests using a serum or urine test with a sensitivity of at least 25 mU/mL; a second test should be performed 8-10 days after the first test and immediately before starting Mycophenolate-Teva.
Repeat pregnancy tests should be performed at standard follow-up visits. The results of all pregnancy tests should be discussed with the patient. Patients should be informed that they should consult with their physician immediately if they become pregnant.
. Because of the mutagenic and teratogenic potential of Mycophenolate-Teva, women of childbearing potential should use two reliable methods of contraception simultaneously (since there is a potential decrease in hormone concentrations when taking oral contraceptives with Mycophenolate-Teva therapy), including at least one highly effective method before starting therapy, during therapy and for six weeks after discontinuing therapy with Mycophenolate-Teva, if abstinence from sexual activity is impossible Sexually active men are advised to use condoms during treatment and for at least 90 days after cessation of therapy.
Condoms should be used by men with normal fertility as well as men after a vasectomy because the risks associated with seminal fluid transmission also apply to men who have had a vasectomy. In addition, female partners of male patients are advised to use highly effective contraceptive methods during treatment and for 90 days after the last dose of Mycophenolate-Teva. During post-registration use, congenital malformations, including multiple malformations, have been identified in children of female patients treated with MMF in combination with other immunosuppressants during pregnancy.
The following malformations were most commonly reported:
The medical literature has reported malformations in 23-27% of live-born children exposed to mycophenolate mofetil during fetal development. By comparison, the risk of malformations in live-born children is approximately 2% in the general population and approximately 4-5% in solid organ transplant patients treated with immunosuppressants other than mycophenolate mofetil.
Patients treated with mycophenolate mofetil have been found to have an increased risk of spontaneous miscarriage, mainly in the first trimester of pregnancy.
The risk in patients treated with mycophenolate mofetil was 45-49%, compared to a rate of 12-33% in patients treated with other immunosuppressants after solid organ transplantation, according to medical literature.
Animal studies have shown reproductive toxicity.
The drug Mycophenolate-Teva is contraindicated during breastfeeding due to the possibility of serious adverse reactions in breastfed children.
In rats, MMF is excreted with milk. Whether MMF is excreted with women’s milk is unknown.
Additional information
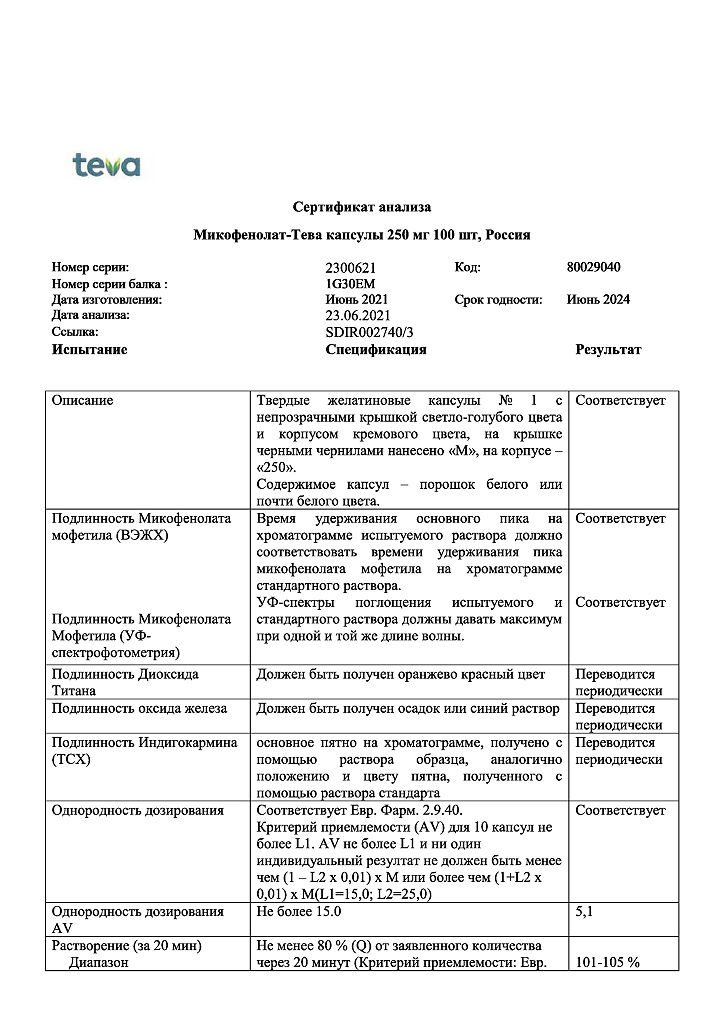
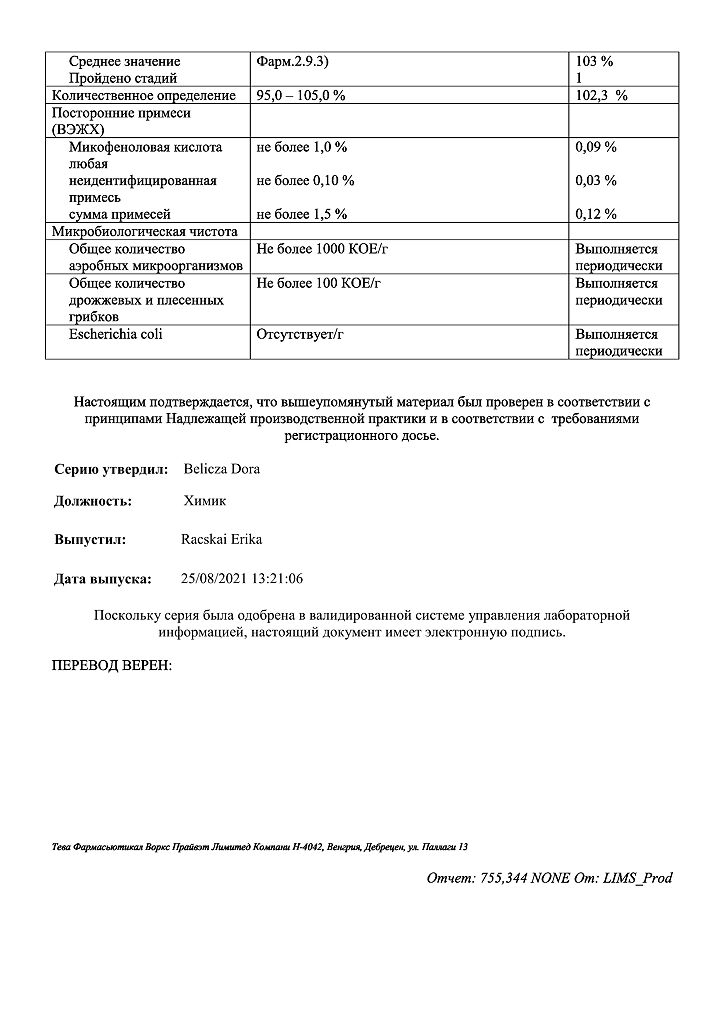
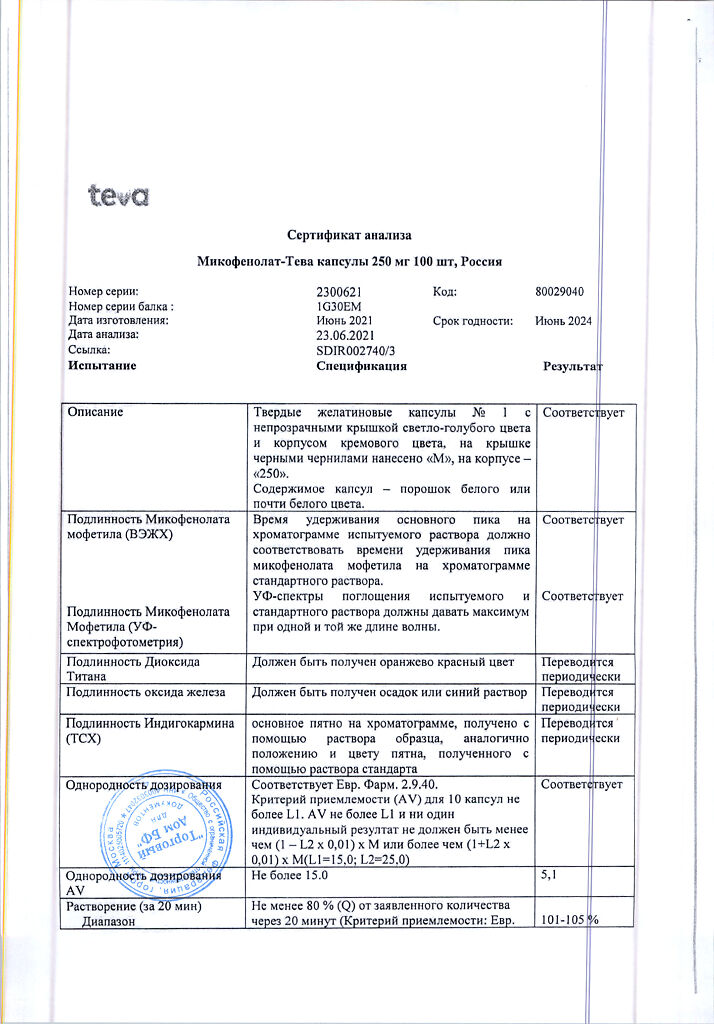
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 oC. Keep out of reach of children! |
| Manufacturer | Teva Pharmaceutical Works Production Limited Company, Hungary |
| Medication form | capsules |
| Brand | Teva Pharmaceutical Works Production Limited Company |
Related products
Buy Mycophenolate-Teva, capsules 250 mg 100 pcs with delivery to USA, UK, Europe and over 120 other countries.