No products in the cart.
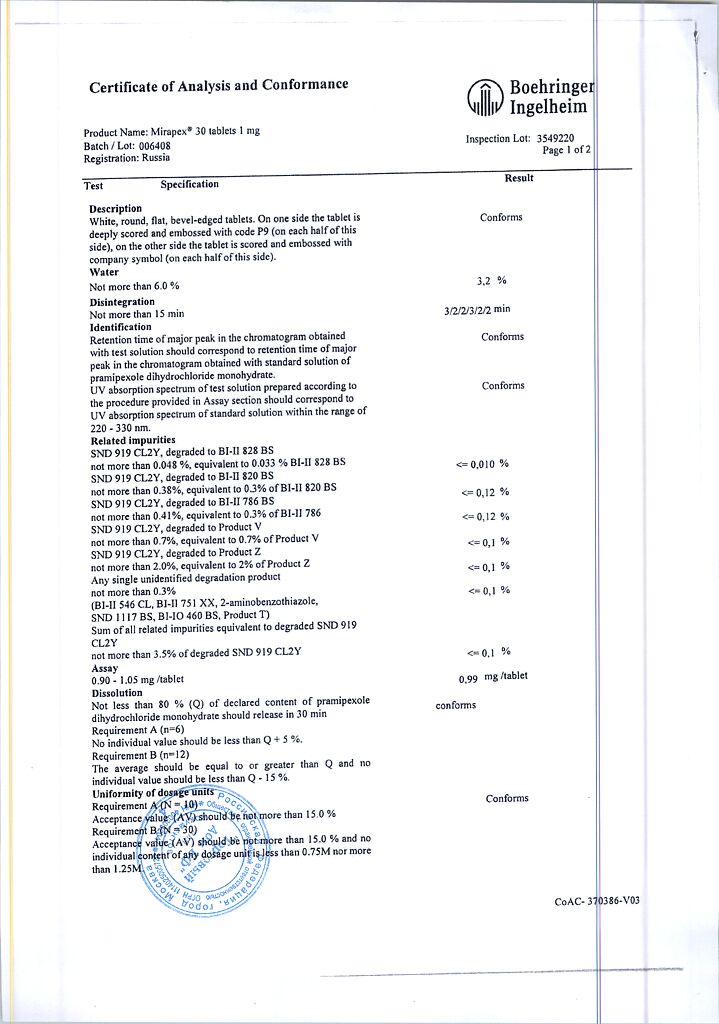
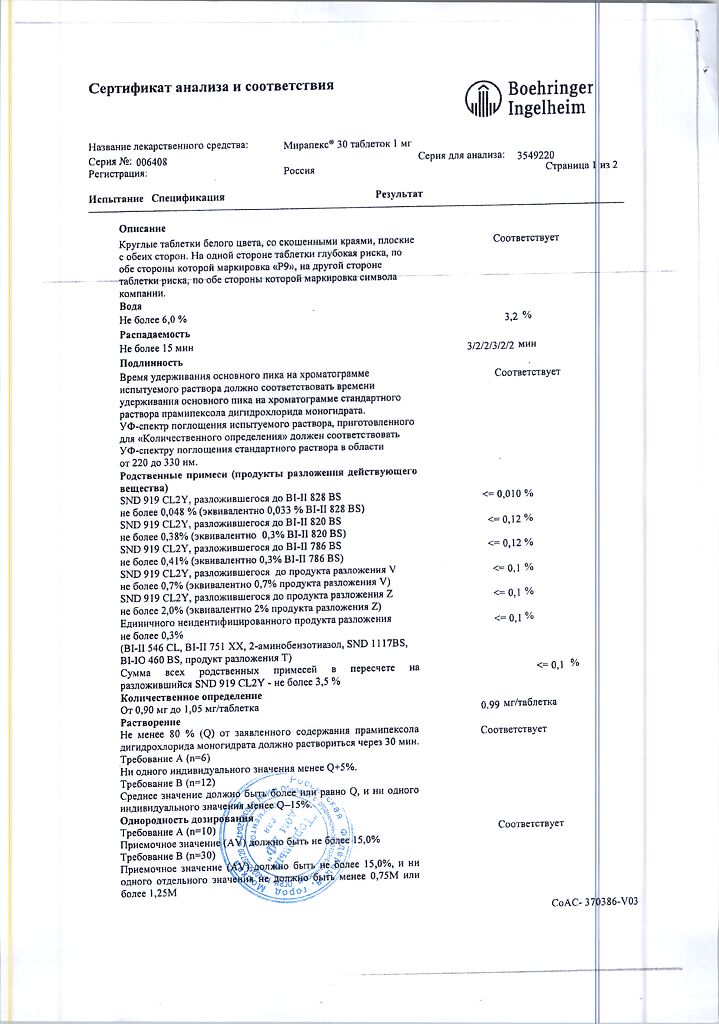
Mirapex, tablets 1 mg 30 pcs
€30.18 €25.15
Description
Pramipexole is a dopaminergic, antiparkinsonian.
Pharmacodynamics
Pramipexole is a dopamine receptor agonist and binds with high selectivity and specificity to dopamine receptors of subgroup D2 group, of which it has the most pronounced affinity for D3 receptors. Reduces motor deficits in Parkinson’s disease by stimulating dopamine receptors in the striatum. Pramipexole inhibits dopamine synthesis, release, and metabolism. In vitro, pramipexole protects dopamine neurons from degeneration in response to ischemia or methamphetamine neurotoxicity.
The drug’s exact mechanism of action in the treatment of restless legs syndrome is currently unknown. Although the pathophysiology of restless legs syndrome is not fully understood, there is neuropharmacological evidence of primary involvement of the dopaminergic system. Studies using positron emission tomography (PET) have shown that moderate presynaptic dopaminergic dysfunction in the striatum may be involved in the pathogenesis of restless legs syndrome.
Pramipexole in vitro protects neurons from levodopa neurotoxicity.
Decreases prolactin secretion (dose-dependent).
In long-term use (>3 years) of pramipexole in patients with Parkinson’s disease, there was no evidence of decreased efficacy.
When pramipexole was used for 1 year in patients with restless legs syndrome, efficacy was maintained.
Pharmacokinetics
Pramipexole is rapidly and completely absorbed after oral administration. Absolute bioavailability is more than 90% and Cmax in plasma is observed after 1-3 hours. Absorption rate is reduced with food, but total absorption is not affected by food intake. Pramipexole is characterized by linear kinetics and relatively little variability in concentrations between patients.
Pramipexole binds very little to proteins (d(400L). It is metabolized to a negligible extent. About 90% of the dose is excreted through the kidneys (80% unchanged) and less than 2% is found in the feces. Total clearance of pramipexole is about 500 ml/min, renal clearance is about 400 ml/min.T1/2 ranges from 8 h in the young to 12 h in the elderly.
Indications
Indications
Symptomatic treatment of idiopathic Parkinson’s disease (monotherapy or in combination with levodopa) and idiopathic restless legs syndrome.
Pharmacological effect
Pharmacological effect
Mirapex – dopaminergic, antiparkinsonian.
Pharmacodynamics
Pramipexole is a dopamine receptor agonist that binds with high selectivity and specificity to dopamine receptors of the D2 subgroup, of which it has the most pronounced affinity for D3 receptors. Reduces motor activity deficits in Parkinson’s disease by stimulating dopamine receptors in the striatum. Pramipexole inhibits the synthesis, release and metabolism of dopamine. Pramipexole in vitro protects dopamine neurons from degeneration that occurs in response to ischemia or methamphetamine neurotoxicity.
The exact mechanism of action of the drug in the treatment of restless legs syndrome is currently unknown. Although the pathophysiology of restless legs syndrome is not fully understood, there is neuropharmacological evidence that the dopaminergic system is primarily involved in the process. Studies using positron emission tomography (PET) have suggested that mild presynaptic dopaminergic dysfunction in the striatum may be involved in the pathogenesis of restless legs syndrome.
Pramipexole protects neurons from levodopa neurotoxicity in vitro.
Reduces the secretion of prolactin (dose-dependent).
With long-term use (more than 3 years) of pramipexole in patients with Parkinson’s disease, there were no signs of decreased effectiveness.
When pramipexole was used in patients with restless legs syndrome for 1 year, the effectiveness of the drug was maintained.
Pharmacokinetics
Pramipexole is rapidly and completely absorbed after oral administration. Absolute bioavailability is more than 90%, and Cmax in plasma is observed after 1-3 hours. The rate of absorption decreases with food intake, but the total amount of absorption is not affected by food intake. Pramipexole exhibits linear kinetics and relatively little interpatient variability in concentrations.
Pramipexole binds to proteins to a very small extent (d(400 l). It is metabolized to a small extent. About 90% of the dose is excreted through the kidneys (80% unchanged) and less than 2% is found in the feces. The total clearance of pramipexole is about 500 ml/min, renal clearance is about 400 ml/min. T1/2 ranges from 8 hours in young people and up to 12 hours in older people.
Special instructions
Special instructions
Hallucinations and confusion are known side effects of dopamine agonist and levodopa treatment. When Mirapex® was used in combination with levodopa in late stages of the disease, hallucinations were observed more often than when using pramipexole monotherapy in patients at an early stage of the disease. Patients should be informed about the possibility of hallucinations (mainly visual), which may affect the ability to drive a car.
Patients and those caring for them should be aware that signs of abnormal behavior (symptoms of impulsive and compulsive actions), such as overeating (hyperphagia), compulsive shopping (pathological shopping), hypersexuality and pathological gambling, may occur in connection with the treatment of patients with dopaminergic drugs. In such cases, a decision should be made to reduce the dose/gradually discontinue treatment.
In patients with psychotic disorders, the prescription of dopamine agonists in combination with pramipexole is possible only after a preliminary assessment of the possible risk-benefit. Concomitant use of pramipexole with antipsychotic drugs should be avoided.
It is recommended to check your vision at regular intervals or immediately after prescribing the drug if such disorders are present.
Caution must be exercised if the patient has severe cardiovascular disease. Due to the risk of orthostatic hypotension during therapy with dopaminergic drugs, it is recommended to monitor blood pressure, especially at the beginning of treatment.
Patients should be warned about the possible sedative effect of the drug. It has been reported that cases of drowsiness and sudden falling asleep during daily activities (including when driving a car or operating complex machinery) may occur at any time during the treatment period, and patients should be informed about this.
Epidemiological studies have shown that patients with Parkinson’s disease have a high risk (2 to approximately 6 times higher) of developing melanoma than the general population. Whether this increased risk is due to Parkinson’s disease or is related to other factors, such as medications used for Parkinson’s disease, is not known.
For the reasons given above, patients and those caring for them should be informed that while taking pramipexole or other dopaminergic drugs they should be alert to the possibility of developing melanoma.
Parkinson’s disease
It was reported that with abrupt cessation of therapy, a symptom complex resembling neuroleptic malignant syndrome was observed.
Increased restless legs syndrome
Reports in the literature suggest that treatment of restless legs syndrome with dopaminergic drugs may lead to an increase in its severity.
This increase represented an earlier onset of symptoms in the evening (or even in the afternoon), an increase in these symptoms, and spread of symptoms to other extremities. However, in a 26-week controlled clinical trial specifically devoted to studying this effect, there was no significant difference in the increase in clinical symptoms between the pramipexole and placebo groups.
Impact on the ability to drive a car and equipment. Patients should be informed of the possibility of hallucinations (mainly visual), which may affect the ability to drive a car.
When using the drug, sedative effects may develop, including drowsiness and falling asleep during everyday activities. Because drowsiness is a common adverse effect with potentially serious consequences, patients should not drive or operate machinery until they have had sufficient experience with Mirapex® to determine whether or not it negatively affects their mental and/or motor performance. If during treatment patients experience increased drowsiness or episodes of falling asleep during daily activities (i.e., while talking, eating, etc.), they should stop driving or operating machinery and consult a doctor.
Active ingredient
Active ingredient
Pramipexole
Composition
Composition
Active ingredient:
pramipexole dihydrochloride monohydrate 1 mg;
Excipients:
mannitol;
corn starch;
colloidal silicon dioxide;
povidone;
magnesium stearate;
Contraindications
Contraindications
hypersensitivity to pramipexole or to any component of the drug;
children’s age (up to 18 years).
With caution: renal failure, decreased blood pressure.
Side Effects
Side Effects
abnormal behavior (symptoms of impulsive and compulsive actions), such as overeating (hyperphagia), compulsive shopping (pathological shopping), hypersexuality and pathological gambling;
abnormal dreams, amnesia, confusion, constipation, delirium, dizziness, dyskinesia, dyspnea, weakness, hallucinations, headache, hyperkinesia, decreased blood pressure, insomnia, libido disorders, nausea, paranoia, peripheral edema;
pneumonia;
itching, rash and other hypersensitivity reactions;
anxiety, drowsiness, sudden falling asleep, fainting, visual disturbances, including decreased visual acuity and clarity of perception, vomiting, changes in body weight.
Interaction
Interaction
Pramipexole to a small extent (
Drugs that inhibit the active secretion of cationic drugs through the renal tubules (for example, cimetidine), or are themselves eliminated by active secretion through the renal tubules, may interact with pramipexole, resulting in a decrease in the clearance of one or both drugs. In case of simultaneous use of such drugs (including amantadine) and pramipexole, it is necessary to pay attention to signs of excessive dopamine stimulation such as dyskinesia, agitation or hallucinations. In such cases, it is necessary to reduce the dose.
Selegiline and levodopa do not affect the pharmacokinetics of pramipexole. Paramipexole does not affect the overall absorption or elimination of levodopa. Interactions with anticholinergic drugs and amantadine have not been studied. However, interaction with amantadine is possible, because drugs have a similar mechanism of elimination. Anticholinergic drugs are primarily metabolized and are therefore unlikely to interact with pramipexole.
When increasing the dose of pramipexole, it is recommended to reduce the dose of levodopa, while the dose of other antiparkinsonian drugs must be maintained at a constant level.
Due to possible cumulative effects, patients should be advised to exercise caution when taking other sedative drugs or alcohol in combination with Mirapex®, as well as when taking drugs that increase the plasma concentration of pramipexole (for example, cimetidine).
Concomitant use of pramipexole with antipsychotics should be avoided (eg, if antagonism is expected).
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 30 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Boehringer Ingelheim Pharma GmbH & Co.KG, Germany
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 30 °C |
| Manufacturer | Boehringer Ingelheim Pharma GmbH & Co. |
| Medication form | pills |
| Brand | #Н/Д |
Other forms…
Related products
Buy Mirapex, tablets 1 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.