No products in the cart.
Micrazyme, capsules 25000 units 50 pcs
€22.77 €19.74
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group Digestive enzyme drug
ATX code: A09AA02.
Pharmacological properties
Mikrazym® is pancreatin pellets in capsules. The composition of the product includes natural enzymes from the animal pancreas – protease, lipase and amylase, which ensure the digestion of proteins, fats and carbohydrates of food.
After taking Mikrazima® the capsule dissolves quickly in the stomach, releasing enteric-coated pancreatin pellets. Due to their small size pellets are quickly and evenly mixed with food and at the same time with the food lump easily penetrate into the duodenum and then into the small intestine where pancreatic enzymes are released and become active, promoting fast and complete digestion of proteins, fats and carbohydrates of food.
The rapid mixing of pancreatin pellets with the stomach contents, their uniform distribution in it, simultaneous passage with the chyme and also preservation of enzymes before their work in the intestine (due to the presence of enteric-soluble coating of pellets) provide higher digestive activity and maximum approaching of the drug to the natural process of digestion.
The enzymatic activity of Micrazyme® is manifested maximum 30 minutes after oral administration, which provides a rapid onset of effect.
After interaction with substrates protease, lipase and amylase in the lower intestine lose activity and are excreted with the intestinal contents. Micrasym® is not absorbed from the gastrointestinal tract and acts only in the intestinal lumen.
Indications
Indications
Replacement therapy for exocrine pancreatic insufficiency:
chronic pancreatitis;
cystic fibrosis;
pancreatic tumors;
surgical interventions on the pancreas.
Symptomatic therapy in complex treatment for the correction of digestive disorders that occur with other diseases or pathological conditions of the gastrointestinal tract:
condition after resection of the stomach, gallbladder, part of the intestine;
diseases or conditions accompanied by a violation of the process of biliary excretion (liver diseases, cholecystitis, gallstones, chronic diseases of the biliary tract, compression of the biliary tract by neoplasms, cystic growths);
intestinal diseases accompanied by impaired movement of intestinal contents.
To improve food digestion in adults and children with normal gastrointestinal function with:
errors in diet (eating fatty foods or large amounts of food, irregular meals);
disorders of chewing function, with a sedentary lifestyle, prolonged immobilization.
Preparation for X-ray and ultrasound examination of the abdominal organs.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group Digestive enzyme agent
ATX code: A09AA02.
Pharmacological properties
Mikrasim® – pancreatin pellets in capsules. The drug contains natural enzymes from the pancreas of animals – protease, lipase and amylase, which ensure the digestion of proteins, fats and carbohydrates of food.
After taking Micrasim®, the capsule quickly dissolves in the stomach, releasing enteric-coated pancreatin pellets. Due to their small size, pellets are quickly and evenly mixed with food and, simultaneously with the food bolus, easily penetrate the duodenum, and then into the small intestine, where pancreatic enzymes are released and begin to actively act, facilitating the rapid and complete digestion of proteins, fats and carbohydrates of food.
Rapid mixing of pancreatin pellets with the contents of the stomach, their uniform distribution in it, simultaneous passage with chyme, as well as the preservation of enzymes before they begin to work in the intestines (due to the presence of an enteric coating of the pellets), ensure higher digestive activity and maximum approximation of the action of the drug to the natural process of digestion.
The enzymatic activity of Mikrazym® appears within 30 minutes after oral administration, which ensures the rapid onset of the effect.
After interaction with substrates, protease, lipase and amylase in the lower intestine lose activity and, together with intestinal contents, are excreted from the body. Mikrasim® is not absorbed from the gastrointestinal tract and acts only in the intestinal lumen.
Special instructions
Special instructions
Children and adults receiving pancreatin therapy in significant doses for a long time should be observed by a specialist.
The main reasons for the ineffectiveness of enzyme therapy are: inactivation of enzymes in the duodenum as a result of acidification of its contents; concomitant diseases of the small intestine (helminthic infestations, dysbiosis); failure of patients to comply with the recommended treatment regimen; use of enzymes that have lost their activity.
Information about the possible effect of the drug on the ability to drive vehicles and machinery
There is no separate data.
Active ingredient
Active ingredient
Pancreatin
Composition
Composition
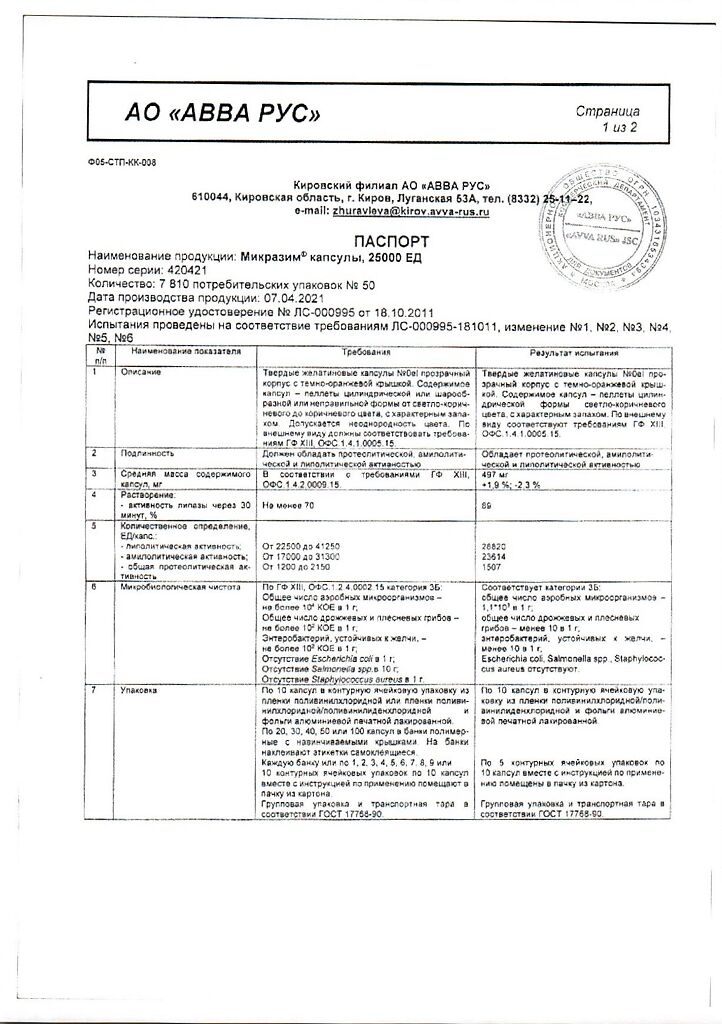
Composition per 1 capsule:
Active substance: 10000IU 25000IU
Pancreatin in the form of enteric pellets, 168 mg* 420 mg*
containing pancreatin powder, 125 mg 312 mg
which corresponds to activity:
protease 520 units 1300 units
amylase 7500 IU 19000 IU
lipase 10,000 units 25,000 units
* – in terms of nominal lipolytic activity.
Excipients included in the enteric pellet shell:
methacrylic acid and ethyl acrylate copolymer [1:1] (in the form of a 30% dispersion additionally containing polysorbate-80, sodium lauryl sulfate) – 25.3 mg / 63.2 mg, triethyl citrate – 5.1 mg / 12.6 mg, simethicone emulsion 30%, dry weight (32.6%) – 0.1 mg / 0.3 mg including:
dimethicone – 27.8%;
colloidal precipitated silicon – 1.3%;
suspended colloidal silicon – 0.9%;
methylcellulose – 2.5%;
sorbic acid – 0.1%;
water – 67.4%, talc – 12.6 mg / 31.6 mg.
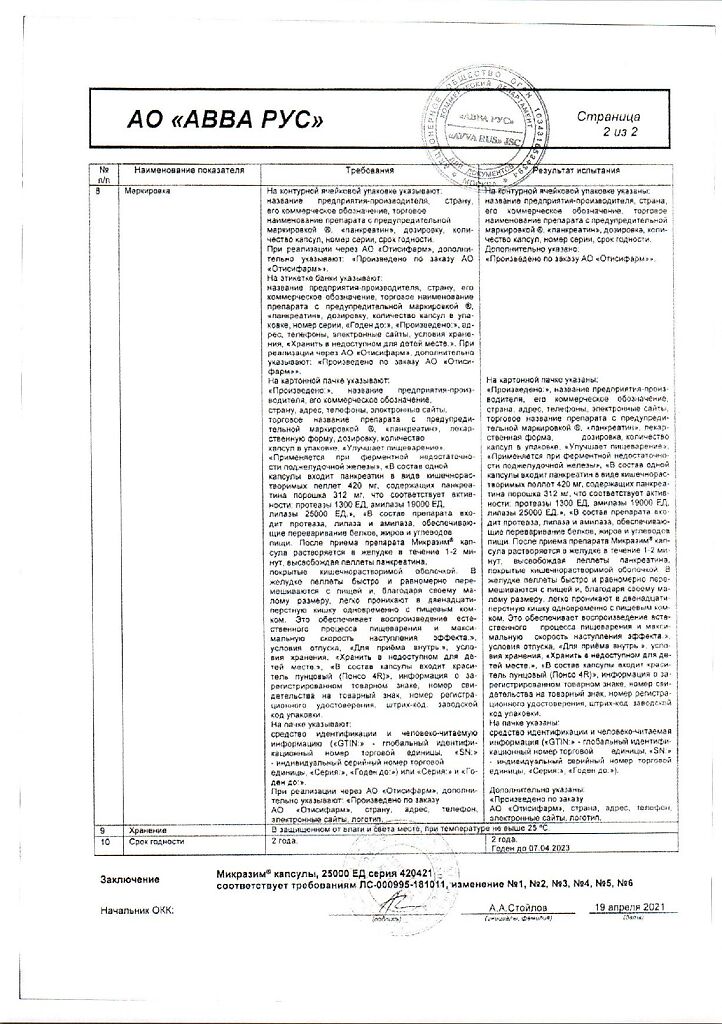
Composition of the capsule shell: body: gelatin – up to 100%, water – 13-16% lid: gelatin – up to 100%, water – 13-16%, crimson dye (Ponceau 4R) – 0.6666% / 0.7999%, quinoline yellow dye – 0.1000% / 0.3166%, patented blue dye – 0.0200% / 0.0053%, titanium dioxide – 1.2999% / 2.9574%.
Pregnancy
Pregnancy
There are no data on the potential risks of using pancreatin during pregnancy and lactation, so the drug should be prescribed to pregnant women and nursing mothers only if the expected effect of therapy outweighs the possible risks.
Contraindications
Contraindications
Individual intolerance to pancreatin or individual components of the drug. Acute pancreatitis, exacerbation of chronic pancreatitis.
Side Effects
Side Effects
Possible: allergic reactions.
Rarely: when used in high doses – diarrhea, nausea, constipation, discomfort in the epigastric area.
With long-term use in high doses: the development of hyperuricosuria and hyperuricemia is possible.
In cystic fibrosis, if the required dose of pancreatin is exceeded, strictures (fibrous colonopathy) may develop in the ileocecal portion of the ascending colon.
Interaction
Interaction
With the simultaneous use of pancreatin with iron preparations, a decrease in iron absorption may occur.
Overdose
Overdose
Symptoms: increased levels of uric acid in the urine (hyperuricuria) and blood (hyperuricemia). Children have constipation.
Treatment: drug withdrawal, symptomatic therapy.
Interactions with other drugs
With the simultaneous use of pancreatin with iron preparations, the absorption of the latter may be reduced.
Storage conditions
Storage conditions
In a place protected from light and moisture, at a temperature not exceeding 25 °C.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
ABVA RUS, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a place protected from light and moisture, at a temperature not exceeding 25 °C. |
| Manufacturer | Avva Rus, Russia |
| Medication form | capsules |
| Brand | Avva Rus |
Other forms…
Related products
Buy Micrazyme, capsules 25000 units 50 pcs with delivery to USA, UK, Europe and over 120 other countries.