No products in the cart.
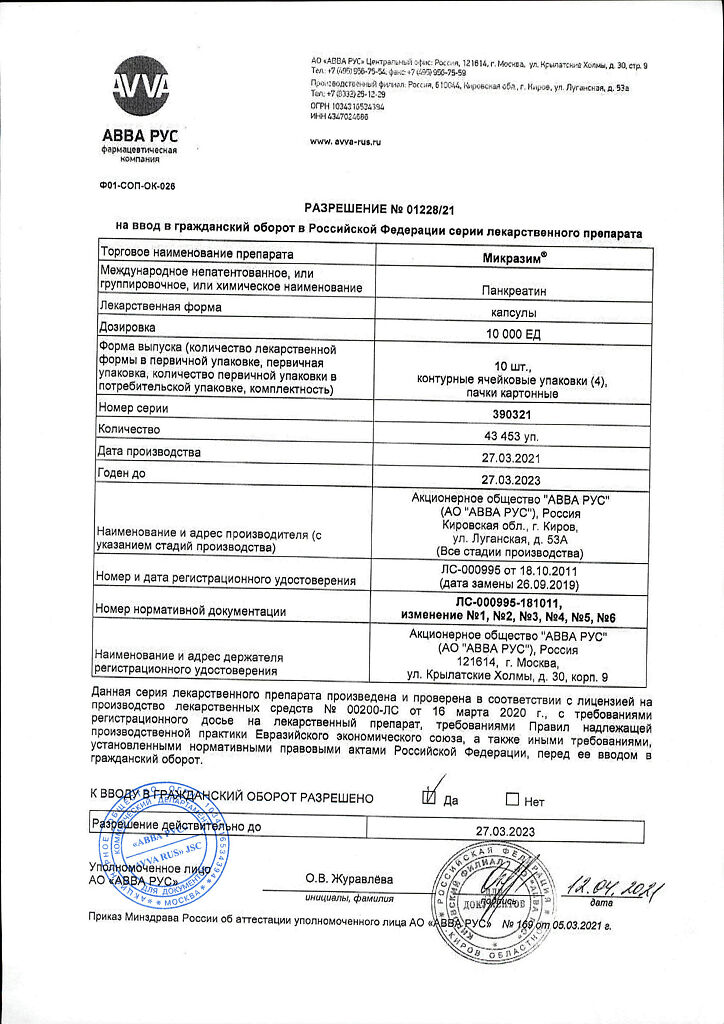
Micrazyme, Capsules 10000 units 20 pcs
€7.53 €6.59
Description
Pharmacotherapeutic group Digestive enzyme drug
ATX code: A09AA02.
Pharmacological properties
Mikrazym® is pancreatin pellets in capsules. The composition of the product includes natural enzymes from the animal pancreas – protease, lipase and amylase, which ensure the digestion of proteins, fats and carbohydrates of food.
After taking Mikrazima® the capsule dissolves quickly in the stomach, releasing enteric-coated pancreatin pellets. Due to their small size pellets are quickly and evenly mixed with food and at the same time with the food lump easily penetrate into the duodenum and then into the small intestine where pancreatic enzymes are released and become active, promoting fast and complete digestion of proteins, fats and carbohydrates of food.
The rapid mixing of pancreatin pellets with the stomach contents, their uniform distribution in it, simultaneous passage with the chyme and also preservation of enzymes before their work in the intestine (due to the presence of enteric-soluble coating of pellets) provide higher digestive activity and maximum approaching of the drug to the natural process of digestion.
The enzymatic activity of Micrazyme® is manifested maximum 30 minutes after oral administration, which provides a rapid onset of effect.
After interaction with substrates protease, lipase and amylase in the lower intestine lose activity and are excreted with the intestinal contents. Micrasym® is not absorbed from the gastrointestinal tract and acts only in the intestinal lumen.
Indications
Indications
Replacement therapy for insufficiency of exocrine pancreatic function in children and adults, caused by various diseases of the gastrointestinal tract, and most often occurring with:
• cystic fibrosis;
• chronic pancreatitis;
• after surgery on the pancreas;
• after gastrectomy;
• pancreatic cancer;
• partial resection of the stomach (for example, Billroth II);
• obstruction of the pancreatic duct or common bile duct (for example, due to a neoplasm);
• Shwachman-Diamond syndrome;
• condition after an attack of acute pancreatitis and the resumption of enteral or oral nutrition.
The drug in a dosage of 10,000 units can be used to improve food digestion in patients with normal gastrointestinal function in cases of dietary errors (eating fatty foods, overeating, irregular meals, etc.).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: digestive enzyme agent
ATX code: A09AA02.
Pharmacological properties
Pharmacodynamics
An enzyme preparation that improves food digestion processes in adults and children, and thereby significantly reduces the symptoms of pancreatic enzyme insufficiency, including abdominal pain, flatulence, changes in stool frequency and consistency. Pancreatic enzymes included in the drug facilitate the breakdown of proteins, fats, and carbohydrates, which leads to their complete absorption in the small intestine.
Mikrasim® contains pancreatin in the form of enteric granules in gelatin capsules. The capsules quickly dissolve in the stomach, releasing granules. This principle is designed to thoroughly mix the granules with chyme, simultaneously transport the granules with chyme from the stomach into the intestines, and, ultimately, better distribute the enzymes after their release within the intestinal contents.
When the granules reach the small intestine, the enteric coating is rapidly destroyed (at pH > 5.5), releasing enzymes with lipolytic, amylolytic and proteolytic activity, resulting in the breakdown of fats, carbohydrates and proteins. The resulting substances are then either absorbed directly or further broken down by intestinal enzymes.
Pharmacokinetics
Animal studies have demonstrated a lack of absorption of uncleaved enzymes and, as a result, classical pharmacokinetic studies have not been performed. Preparations containing pancreatic enzymes do not require absorption to produce their effects. On the contrary, the therapeutic activity of these drugs is fully realized in the lumen of the gastrointestinal tract. According to their chemical structure, they are proteins and, in connection with this, when passing through the gastrointestinal tract, enzyme preparations are broken down until absorption occurs in the form of peptides and amino acids.
Special instructions
Special instructions
In patients with cystic fibrosis who received high doses of pancreatin preparations, strictures of the ileum, cecum and colon (fibrosing colonopathy) have been described. As a precaution, if unusual symptoms or changes in the abdominal cavity occur, medical examination is necessary to rule out fibrosing colonopathy, especially in patients taking the drug at a dose of more than 10,000 lipase units/kg per day.
To avoid complications, use only after consulting a doctor.
Impact on the ability to drive vehicles and machinery
The use of the drug Mikrasim® does not affect or has an insignificant effect on the ability to drive a car and operate machinery.
Active ingredient
Active ingredient
Pancreatin
Composition
Composition
Composition per capsule:
Active ingredient:
10000 units
25000 units
Pancreatin in the form of enteric pellets,
173 mg*
432 mg*
containing pancreatin powder,
125 mg
312 mg
which corresponds to activity:
amylase
7500 units
19000 units
lipases
10000 units
25000 units
proteases
520 units
1300 units
*- in terms of nominal activity.
Excipients:
Methacrylic acid and ethyl acrylate copolymer [1:1] (in the form of a 30% dispersion additionally containing polysorbate-80, sodium lauryl sulfate) – 26.0 mg / 64.9 mg, Talc – 13.0 mg / 32.5 mg, Triethyl citrate – 5.2 mg / 13.0 mg, Poloxamer 407 – 3.7 mg /
9.3 mg; Simethicone emulsion 30%, dry weight (32.6%) – 0.1 mg / 0.3 mg including:
water – 67.4%; dimethicone – 27.8%; methylcellulose – 2.5%; colloidal precipitated silicon – 1.3%; suspended colloidal silicon – 0.9%; sorbic acid – 0.1%.
Hard gelatin capsules:
Body: gelatin – up to 100%, water – 13-16%; Cover: gelatin – up to 100%, water – 13-16%, titanium dioxide – 1.2999% / 2.9574%, crimson dye (Ponceau 4R) – 0.6666% /
0.7999%, quinoline yellow dye – 0.1000% / 0.3166%, patent blue dye – 0.0200% / 0.0053%.
Pregnancy
Pregnancy
Pregnancy
There are no clinical data on the treatment of pregnant women with drugs containing pancreatic enzymes. Animal studies have not demonstrated absorption of pancreatic enzymes of porcine origin and therefore no toxic effects on reproductive function or fetal development are expected.
The drug should be prescribed to pregnant women with caution if the expected benefit to the mother outweighs the potential risk to the fetus.
Breastfeeding period
Based on animal studies in which no systematic negative effects of pancreatic enzymes were observed, no harmful effects of the drug on the nursing infant through breast milk are expected.
During breastfeeding, you can take pancreatic enzymes.
If necessary, during pregnancy or during breastfeeding, the drug should be taken in doses sufficient to maintain adequate nutritional status.
Contraindications
Contraindications
Hypersensitivity to the active substance of the drug or to any excipient.
Side Effects
Side Effects
In clinical studies of pancreatin, more than 1000 patients were treated.
The most common adverse reactions were gastrointestinal disorders, which were mostly mild or moderate in severity.
In clinical studies, the following adverse reactions were reported with the indicated frequencies:
System
organs
Very common: ≥1/10
Common: ≥ 1/100 to < 1/10
Uncommon: ≥ 1/1000 to < 1/100
Frequency unknown
Violations with
sides
gastro-
intestinal
tract
abdominal pain*
nausea, vomiting, constipation, bloating, diarrhea*
strictures
ileum, cecum, or colon (fibrosing colonopathy)
Skin and subcutaneous tissue disorders
rash
itching, urticaria
Immune system disorders
hypersensitivity
(anaphylactic
reactions)
* Gastrointestinal disorders are mainly related to the underlying disease. The incidence of adverse reactions such as abdominal pain and diarrhea was lower or similar to that observed with placebo.
Strictures of the ileum, cecum, or colon (fibrosing colonopathy) have been observed in patients with cystic fibrosis receiving high doses of pancreatin preparations (see section “Special Instructions”).
Allergic reactions were observed mainly from the skin, but other manifestations of allergies were also noted. Reports of these side effects were received during post-marketing use and were spontaneous. There are insufficient data to accurately estimate the incidence of cases.
When used in children, no specific adverse reactions were observed. The frequency, type and severity of adverse reactions in children with cystic fibrosis were similar to those in adults.
Interaction
Interaction
No interaction studies have been conducted.
Overdose
Overdose
Symptoms: hyperuricosuria and hyperuricemia.
Treatment: drug withdrawal, symptomatic therapy.
Storage conditions
Storage conditions
In the original packaging (pack) at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use after expiration date.
Manufacturer
Manufacturer
ABVA RUS, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a place protected from light and moisture, at a temperature not exceeding 25 °C. |
| Manufacturer | Avva Rus, Russia |
| Medication form | capsules |
| Brand | Avva Rus |
Other forms…
Related products
Buy Micrazyme, Capsules 10000 units 20 pcs with delivery to USA, UK, Europe and over 120 other countries.