No products in the cart.
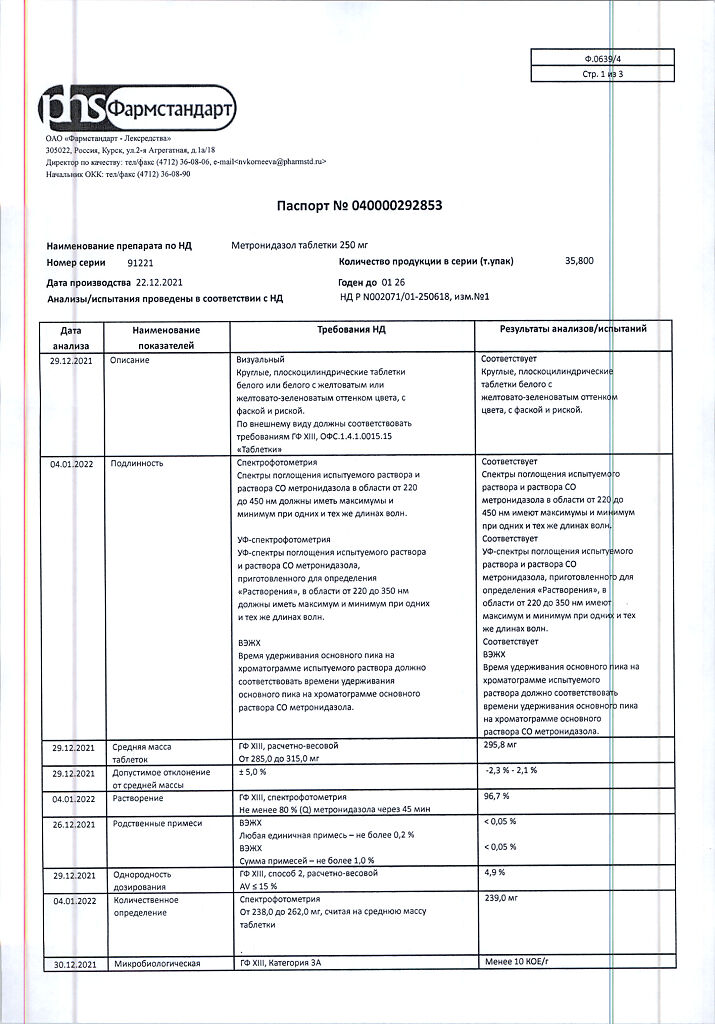
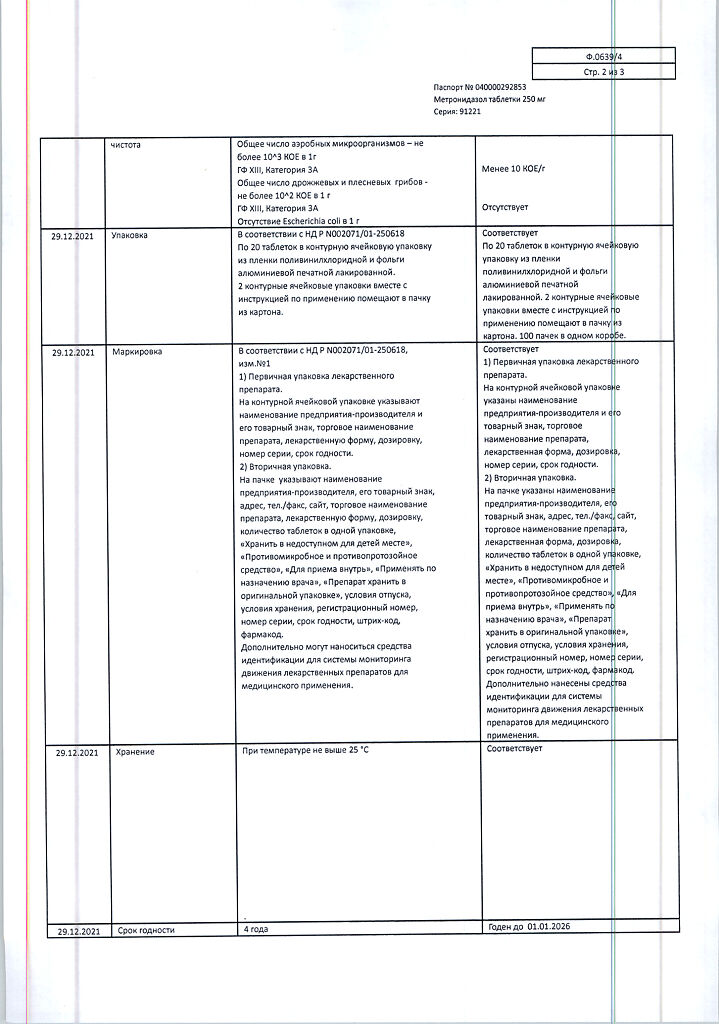
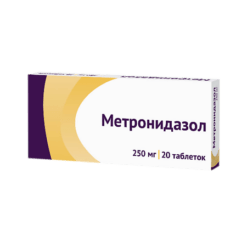
Metronidazole, tablets 250 mg 40 pcs
€4.45 €3.96
Description
Metronidazole refers to 5-nitroimidazole and has a broad spectrum of action against anaerobic microorganisms Peptostreptococcus species, Clostridium spp., Bacterioides spp., Fusobacterium spp., Veillonella spp., Prevotella (Prevotella bivia, Prevotella buccae, Prevotella disiens).
Indications
Indications
Trichomonas vaginitis and urethritis in women, Trichomonas urethritis in men, giardiasis, amoebic dysentery; anaerobic infections caused by microorganisms sensitive to the drug. Combination therapy for severe mixed aerobic-anaerobic infections. Prevention of anaerobic infection during surgical interventions (especially on the abdominal organs, urinary tract).
In combination with amoxicillin: chronic gastritis in the acute phase, peptic ulcer of the stomach and duodenum in the acute phase, associated with Helicobacter pylori.
Pharmacological effect
Pharmacological effect
Metronidazole belongs to the 5-nitroimidazoles and has a wide spectrum of action against anaerobic microorganisms Peptostreptococcus species, Clostridium spp., Bacterioides spp., Fusobacterium spp., Veillonella spp., Prevotella (Prevotella bivia, Prevotella buccae, Prevotella disiens).
Special instructions
Special instructions
During the treatment period, ethanol intake is contraindicated (a disulfiram-like reaction may develop: cramping abdominal pain, nausea, vomiting, headache, sudden rush of blood to the face).
In combination with amoxicillin, it is not recommended for use in patients under 18 years of age.
With long-term therapy, it is necessary to monitor the blood picture. With leukopenia, the possibility of continuing treatment depends on the risk of developing an infectious process.
The appearance of ataxia, dizziness and any other deterioration in the neurological status of patients requires cessation of treatment.
May immobilize treponemes and lead to a false-positive Nelson test.
Colors urine dark.
When treating trichomonas vaginitis in women and trichomonas urethritis in men, it is necessary to abstain from sexual activity. Simultaneous treatment of sexual partners is mandatory. Treatment does not stop during menstruation. After treatment for trichomoniasis, control tests should be carried out during three consecutive cycles before and after menstruation.
After treatment of giardiasis, if symptoms persist, after 3-4 weeks, carry out 3 stool tests at intervals of several days (in some successfully treated patients, lactose intolerance caused by infestation may persist for several weeks or months, resembling the symptoms of giardiasis).
During the treatment period, it is recommended to stop breastfeeding.
Active ingredient
Active ingredient
Metronidazole
Composition
Composition
Composition per tablet:
active substance:
metronidazole – 250 mg;
excipients:
potato starch – 45.0 mg,
povidone (low molecular weight medical polyvinylpyrrolidone) – 2.0 mg,
stearic acid – 3.0 mg.
Contraindications
Contraindications
Hypersensitivity to any of the components of the drug and imidazoles;
organic lesions of the central nervous system (including epilepsy);
pregnancy and lactation;
blood diseases, leukopenia (including history);
children under 3 years of age (for this dosage form).
WITH CAUTION
Renal and/or liver failure.
Side Effects
Side Effects
Allergic reactions: urticaria, skin rash.
Local reactions: burning sensation or irritation of the penis in a sexual partner, burning sensation or increased urination, vulvitis (itching, burning pain or hyperemia of the mucous membrane in the external genital area). After discontinuation of the drug, vaginal candidiasis may develop.
Systemic reactions: possible changes in taste sensations, including a metallic taste, dizziness, headache, dry mouth, nausea, vomiting, decreased appetite, abdominal cramps, constipation or diarrhea, dark urine, leukopenia or leukocytosis.
Interaction
Interaction
When used simultaneously with antacids containing aluminum hydroxide and cholestyramine, the absorption of metronidazole from the gastrointestinal tract is slightly reduced.
With simultaneous use, metronidazole potentiates the effect of indirect anticoagulants.
When used simultaneously with disulfiram, the development of acute psychosis and impaired consciousness is possible.
An increase in the concentration of carbamazepine in the blood plasma and an increase in the risk of developing toxic effects cannot be excluded when used simultaneously with metronidazole.
When used simultaneously with lansoprazole, glossitis, stomatitis and/or the appearance of a dark color of the tongue are possible; with lithium carbonate – it is possible to increase the concentration of lithium in the blood plasma and develop symptoms of intoxication; with prednisone – the excretion of metronidazole from the body increases due to the acceleration of its metabolism in the liver under the influence of prednisone. The effectiveness of metronidazole may be reduced.
When used simultaneously with rifampicin, the clearance of metronidazole from the body increases; with phenytoin – a slight increase in the concentration of phenytoin in the blood plasma is possible; a case of toxic effects has been described.
When used simultaneously with phenobarbital, the excretion of metronidazole from the body significantly increases, apparently due to the acceleration of its metabolism in the liver under the influence of phenobarbital. The effectiveness of metronidazole may be reduced.
When used simultaneously with fluorouracil, the toxic effect, but not the effectiveness of fluorouracil, increases.
A case of the development of acute dystonia after taking a single dose of chloroquine in a patient receiving metronidazole is described.
When used simultaneously with cimetidine, inhibition of the metabolism of metronidazole in the liver is possible, which can lead to a slower elimination and increased concentration in the blood plasma.
With simultaneous use of ethanol in patients receiving metronidazole, disulfiram-like reactions may develop.
Overdose
Overdose
Symptoms: nausea, vomiting, ataxia; when taken as a radiosensitizing agent – convulsions, peripheral neuropathy.
Treatment: no specific antidote, symptomatic and supportive therapy.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
4 years. Do not use the drug after the expiration date.
Manufacturer
Manufacturer
Pharmstandard-Leksredstva, Russia
Additional information
| Shelf life | 4 years. Do not use the drug after the expiration date. |
|---|---|
| Conditions of storage | Store at the temperature not more than 25 °С. Keep out of reach of children. |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | pills |
| Brand | Pharmstandard-Leksredstva |
Other forms…
Related products
Buy Metronidazole, tablets 250 mg 40 pcs with delivery to USA, UK, Europe and over 120 other countries.