No products in the cart.
Metrogil, 5 mg/ml 100 ml
€0.92 €0.84
EAN: 8901086130013
SKU: 29917
Categories: Gynecology and Obstetrics, Medicine, Trichomoniasis and malaria
Description
Metrogil has antibacterial, antiprotozoal (trichomonacid) action.
The nitrogroup of metronidazole is reduced in anaerobic microorganisms and protozoa and interacts with the DNA of the microbial cell, inhibiting the synthesis of their nucleic acids, which leads to the death of the bacteria.
Metronidazole is active against Trichomonas vaginalis, Gardnerella vaginalis, Giardia intestinalis, Entamoeba histolytica, Lamblia, anaerobic bacteria: Bacteroides spp.
In combination with amoxicillin it shows activity against Helicobacter pylori (amoxicillin suppresses the development of resistance to metronidazole).
Metronidazole is insensitive to aerobic microorganisms and facultative anaerobes, but in the presence of mixed flora (aerobes and anaerobes) metronidazole acts synergistically with antibiotics effective against common aerobes.
It increases the sensitivity of tumors to radiation, causes disulfiram-like reactions and stimulates reparative processes.
When applied topically, it has an anti-acne effect, the exact mechanism of which is unknown.
Indications
Indications
prevention and treatment of anaerobic infections during surgical interventions mainly on the abdominal organs and urinary tract;
severe mixed aerobic-anaerobic infections (combination therapy), intestinal and hepatic amebiasis, sepsis, peritonitis, osteomyelitis, gynecological infections, abscesses of the pelvic organs and brain, abscess pneumonia, gas gangrene, infections of the skin and soft tissues, bones and joints.
Radiation therapy for patients with tumors – as a radiosensitizing drug in cases where tumor resistance is due to hypoxia in tumor cells.
Pharmacological effect
Pharmacological effect
Metrogyl has an antibacterial, antiprotozoal (trichomonacid) effect.
The nitro group of metronidazole is restored in anaerobic microorganisms and protozoa, interacts with the DNA of the cell of microorganisms, inhibiting the synthesis of their nucleic acids, which leads to the death of bacteria.
Metronidazole is active against Trichomonas vaginalis, Gardnerella vaginalis, Giardia intestinalis, Entamoeba histolytica, Lamblia, anaerobic bacteria: Bacteroides spp., Fusobacterium spp.
In combination with amoxicillin, it is active against Helicobacter pylori (amoxicillin suppresses the development of resistance to metronidazole).
Aerobic microorganisms and facultative anaerobes are insensitive to metronidazole, but in the presence of mixed flora (aerobes and anaerobes), metronidazole acts synergistically with antibiotics that are effective against common aerobes.
Increases the sensitivity of tumors to radiation, causes disulfiram-like reactions, and stimulates reparative processes.
When applied topically, it has an anti-acne effect, the mechanism of which is precisely unknown.
Special instructions
Special instructions
During long-term treatment, systematic monitoring of peripheral blood patterns is necessary. With leukopenia, the possibility of continuing treatment depends on the danger of the infectious process.
Treatment should be stopped if ataxia, dizziness, or deterioration of the neurological status of patients occur.
It must be taken into account that metronidazole can immobilize treponemes, which leads to a false-positive Nelson test.
During use of the drug, a darker coloration of urine is observed.
In combination with amoxicillin, it is not recommended for use in patients under 18 years of age; should not be used for gastrointestinal diseases with prolonged diarrhea or vomiting; Prescribed with caution for liver diseases.
Active ingredient
Active ingredient
Metronidazole
Composition
Composition
Active ingredient:
metronidazole 5 mg
Contraindications
Contraindications
Children under 6 years of age; individual intolerance to metronidazole, chlorhexidine, as well as nitroimidazole derivatives and other components of the drug.
Restrictions on use:
Pregnancy, lactation period.
Diseases of the liver with impairment of its functions (cumulation is possible), central and peripheral nervous system, blood diseases.
During treatment you should not drink alcohol.
Side Effects
Side Effects
From the digestive system: diarrhea, loss of appetite, nausea, vomiting, intestinal colic, constipation, “metallic” taste in the mouth, dry mouth, glossitis, stomatitis, pancreatitis.
From the nervous system: dizziness, impaired coordination of movements, ataxia, confusion, irritability, depression, increased excitability, weakness, insomnia, headache, convulsions, hallucinations, peripheral neuropathy.
Allergic reactions: urticaria, skin rash, skin hyperemia, nasal congestion, fever, arthralgia.
From the urinary system: dysuria, cystitis, polyuria, urinary incontinence, candidiasis, red-brown coloration of urine.
Local reactions: thrombophlebitis (pain, hyperemia or swelling at the injection site).
Other: neutropenia, leukopenia, flattening of the T wave on the ECG.
Interaction
Interaction
Strengthens the effect of indirect anticoagulants, which leads to an increase in the time of prothrombin formation. Similar to disulfiram, it causes ethanol intolerance.
Concomitant use with disulfiram can lead to the development of various neurological symptoms (the interval between prescriptions is at least 2 weeks).
Metronidazole for intravenous administration is not recommended to be mixed with other drugs.
Cimetidine inhibits the metabolism of metronidazole, which may lead to an increase in its concentration in the blood serum and an increased risk of side effects.
The simultaneous administration of drugs that stimulate microsomal oxidation enzymes in the liver (phenobarbital, phenytoin) can accelerate the elimination of metronidazole, resulting in a decrease in its plasma concentration.
When taken simultaneously with Li+ drugs, the concentration of the latter in the plasma may increase and the development of symptoms of intoxication.
It is not recommended to combine with non-depolarizing muscle relaxants (vecuronium bromide). Sulfonamides enhance the antimicrobial effect of metronidazole.
Overdose
Overdose
Symptoms: nausea, vomiting, ataxia, in severe cases – peripheral neuropathy and epileptic seizures.
Treatment: symptomatic; There are no specific antidotes.
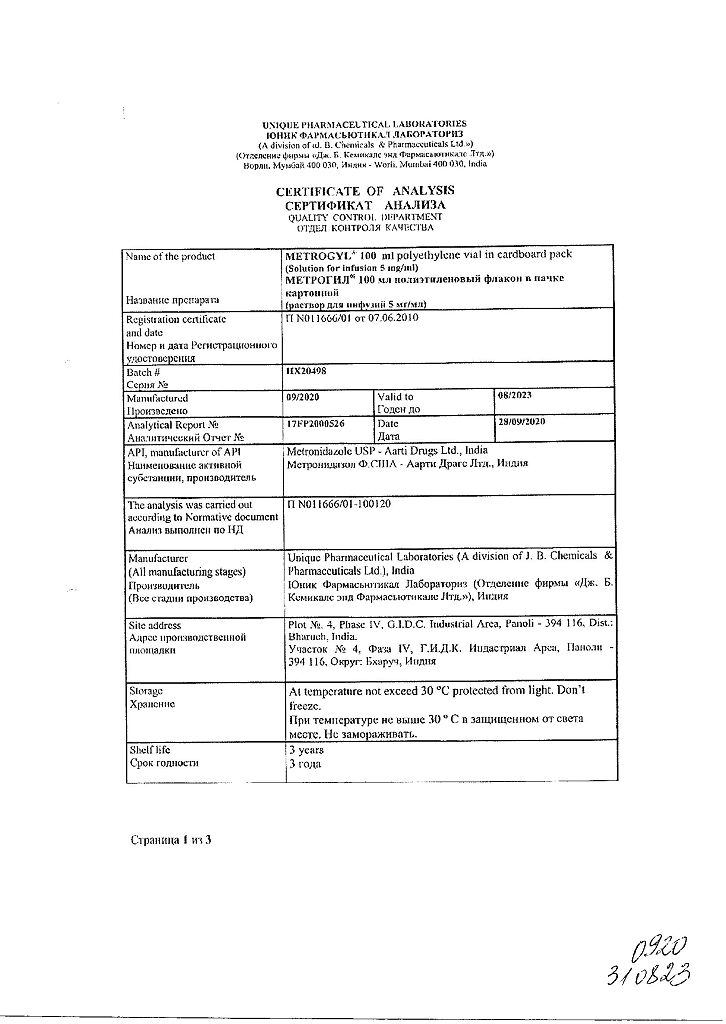
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 30 °C (do not freeze)
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Unique Pharmaceutical Laboratories, India
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In the dark place at a temperature not exceeding 30 °C (do not freeze) |
| Manufacturer | Unique Pharmaceutical Laboratories, India |
| Medication form | solution for infusion |
| Brand | Unique Pharmaceutical Laboratories |
Other forms…
Related products
Gynecology and Obstetrics
Buy Metrogil, 5 mg/ml 100 ml with delivery to USA, UK, Europe and over 120 other countries.