No products in the cart.
Meloxicam-Vertex, tablets 15 mg 20 pcs
€9.51 €8.32
Description
Pharmgroup:
NSAIDs.
Pharmacology
The mechanism of action
Meloxicam is a NSAID that shows anti-inflammatory, analgesic and antipyretic effects in animal models. The mechanism of action of meloxicam, like other NSAIDs, is associated with COX inhibition.
Like other NSAIDs, meloxicam competitively inhibits both COX isoenzymes, COX-1 and COX-2, by blocking arachidonate metabolism, resulting in analgesic, antipyretic and anti-inflammatory pharmacological effects. NSAIDs do not affect the peroxidase response and do not inhibit the synthesis of leukotrienes by lipoxygenase pathways. Meloxicam has a predominant effect on COX-2.
Gastrointestinal side effects of meloxicam are mainly related to COX-1 inhibition, but the potential role of COX-2 inhibition in the GI tract is not fully understood.
In the kidneys, PGs produced by both COX-1 and COX-2 are important regulators of sodium and water metabolism as well as renal function and hemodynamics. In conditions where the renal blood flow depends on the synthesis of COX, taking NSAIDs can lead to a significant decrease in renal blood flow, which can cause acute renal failure. In addition, changes in sodium and water reabsorption can worsen the condition of patients with elevated BP.
Pharmacokinetics
Intake
The absolute bioavailability of meloxicam capsules was 89% after a single oral dose of 30 mg compared to a bolus IV injection at the same dose. Dose-dependent pharmacokinetics were observed in the dose range from 5 to 60 mg after a single IV injection. After multiple oral doses, the pharmacokinetics of meloxicam in capsules was dose-dependent over a dose range of 7.5 to 15 mg. Mean Cmax was reached within 4-5 h after ingestion of meloxicam 7.5 mg tablet on an empty stomach, indicating prolonged absorption of the drug. With multiple dosing, stable concentrations were achieved by day 5. A second peak in meloxicam concentrations was observed approximately 12-14 h after administration of the dose, indicating intrahepatic recirculation.
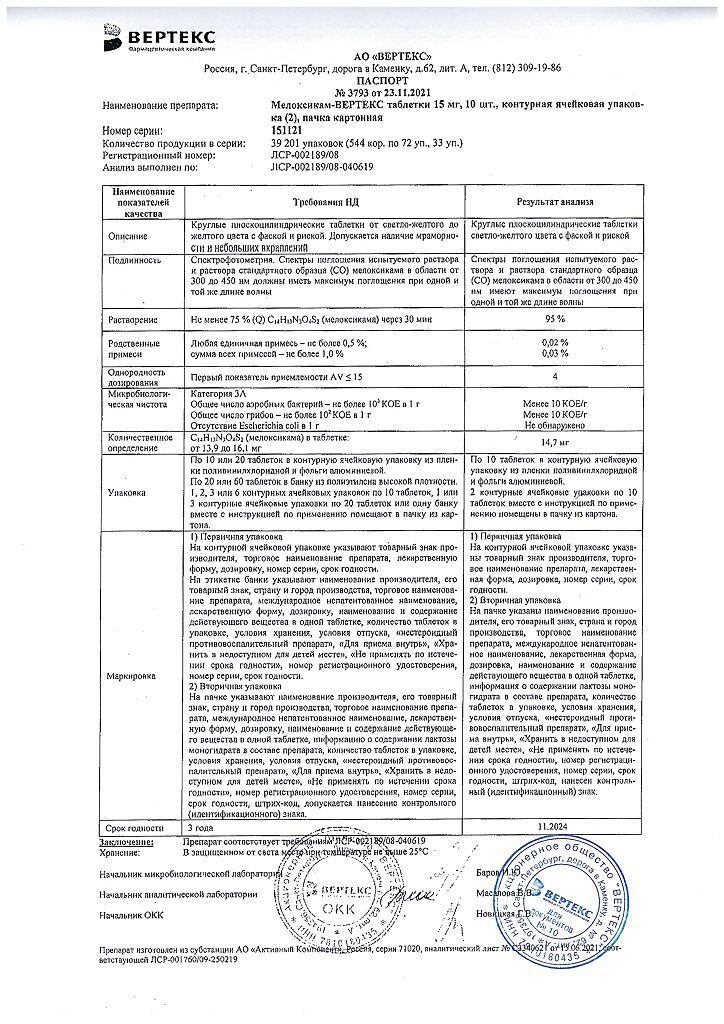
Table 1
Pharmacokinetic parameters of single dose and equilibrium states with oral administration of meloxicam at doses of 7.5 and 15 mg (mean value and coefficient of variation, %)
/p>
| Pharmacokinetic parameter1 | Equilibrium state | Single dose | ||||
| Healthy adult men, 7.5 mg tablets, after a low-fat meal (n=18) | Older men, 15 mg capsules, after a low-fat meal (n=5) | Elderly women, 15 mg capsules, after a low-fat meal (n=8) | Renal failure, 15 mg capsules, fasting (n=12) | Liver failure, 15 mg capsules, fasting (n=12) | ||
| Cmax, µg/ml | 1.05 (20) | 2.3 (59) | 3.2 (24) | 0.59 (36) | 0.84 (29) | |
| Tmax, h | 4.9 (8) | 5 (12) | 6 (27) | 4 (65) | ||
| T1/2, h | 20.1 (29) | 21 (34) | 24 (34) | 18 (46) | 16 (29) | |
| CL/f, ml/min | 8.8 (29) | 9.9 (76) | 5.1 (22) | 19 (43) | 11 (44) | |
| Vz/f (dose/(AUC-Kel), l | 14.7 (32) | 15 (42) | 10 (30) | 26 (44) | 14 (29) | |
1 The parameter values in the table are taken from various studies.
Ingestion of food and antacids. Administration of meloxicam capsules after a high-fat breakfast (75 g fat) resulted in an increase in Cmax of approximately 22%, while AUC was unchanged. Tmax was 5 to 6 h. No FCV was detected with concomitant administration of antacids. Based on these data, meloxicam tablets can be administered regardless of the time of eating or concomitant use of antacids.
Distribution
The average Vss of meloxicam is approximately 10L. Meloxicam is approximately 99.4% bound to plasma proteins (primarily to albumin ) in the therapeutic dose range. The proportion of binding to proteins does not depend on the concentration of meloxicam in the clinically relevant range, but decreases to about 99% in patients with renal insufficiency. Meloxicam penetration into human erythrocytes after oral administration is less than 10%. After administration of a radioactively labeled dose, more than 90% of the radioactivity detected in plasma was present as unchanged meloxicam.
The concentration of meloxicam in synovial fluid after a single oral administration is 40 to 50% of the plasma concentration. The free fraction in synovial fluid is 2.5 times higher than in plasma due to the lower albumin content in synovial fluid compared to plasma. The significance of this phenomenon is unknown.
Metabolism
Meloxicam is almost completely metabolized to four pharmacologically inactive metabolites. The main metabolite, 5′-carboxymeloxicam (60% of the dose), is formed by oxidation of the intermediate metabolite 5′-hydroxymethylmeloxicam, which is excreted at 9% of the dose. In vitro studies show that CYP2C9 plays an important role in this metabolic pathway with a minor contribution of CYP3A4 isoenzyme. Peroxidase activity is probably responsible for the other two metabolites, which account for 16 and 4% of the administered dose, respectively.
The excretion of meloxicam occurs predominantly as metabolites and equally in the urine and feces. Only traces of the unchanged parent compound are excreted with urine (0.2%) and feces (1.6%). Urinary excretion was confirmed for unchanged multiple doses of 7.5 mg – 0.5%, 6% and 13% were detected in the urine as meloxicam, 5′-hydroxymethyl- and 5′-carboxymetabolites, respectively. Significant biliary and/or enteric secretion of meloxicam has been observed.
This has been demonstrated when oral administration of colestyramine after a single IV injection of meloxicam reduced its AUC by 50%. The average T1/2 is 15 to 20 h. T1/2 is unchanged at different dose levels, indicating a linear metabolism over a range of therapeutic doses. Plasma clearance is 7 to 9 ml/min.
Particular patient groups
Elderly age. In elderly men (≥65 years), plasma concentrations of meloxicam and equilibrium pharmacokinetics were similar to those observed in younger patients. In older women (≥65 years), after weight normalization, AUCss by 47% and Cmax, ss by 32% were higher than in younger women (≤55 years). Despite this increase, the adverse event profile was comparable for both patient groups. A lower free fraction was found in older female patients compared with older male patients.
Gender. Young women have slightly lower plasma concentrations than young men. After a single dose of 7.5 mg of meloxicam, the mean T1/2 was 19.5 h for women compared with 23.4 h for men. In the equilibrium state, the data were similar (17.9 versus 21.4 h). This difference by sex is probably of little clinical significance. Linearity in pharmacokinetics was observed and there were no appreciable differences in Cmax or Tmax values for men and women.
Hepatic impairment. After a single dose of meloxicam 15 mg, there were no observable differences in plasma concentrations in patients with mild (Child-Pugh Class A) and moderate (Child-Pugh Class B) hepatic impairment compared to healthy volunteers. Hepatic insufficiency did not affect the binding of meloxicam to plasma proteins. No dose adjustment is required in mild to moderate hepatic impairment. Sufficient studies with the participation of patients with severe hepatic impairment (class C according to Child-Pugh classification) have not been conducted.
Renal insufficiency. Pharmacokinetics of meloxicam has been studied in patients with renal insufficiency of various degrees. Total plasma concentration of the drug decreased according to the severity of renal failure, while the AUC value of the free fraction was similar. Total clearance of meloxicam was increased in patients with renal failure, probably due to an increase in the free fraction, which resulted in accelerated metabolic clearance.
In patients with mild to moderate renal impairment (creatinine Cl >15 ml/min), no dose adjustment is required. Patients with severe renal impairment have not been studied sufficiently. The use of meloxicam in patients with severe renal impairment is not recommended.
Hemodialysis. After a single dose of meloxicam, the Cmax of free fraction in plasma was higher in patients with renal failure who were on permanent hemodialysis (free fraction 1%) compared to healthy volunteers (0.3%). Hemodialysis did not decrease the total plasma concentration of meloxicam. Thus, no additional doses are required after hemodialysis. Meloxicam is not amenable to dialysis.
Clinical studies
Osteoarthritis and rheumatoid arthritis. The use of meloxicam for the treatment of signs and symptoms of osteoarthritis of the knee and hip joints was evaluated in a 12-week double-blind controlled study. Meloxicam (3.75, 7.5, and 15 mg/d) was compared with placebo. The four primary endpoints were the investigator’s total score, the patient’s total score, the patient’s pain score, and the total WOMAC score (a self-administered questionnaire concerning pain, function, and stiffness). Patients receiving meloxicam at doses of 7.5 and 15 mg/day showed significant improvement in each of these endpoints compared to placebo.
The use of meloxicam to treat signs and symptoms of osteoarthritis has been evaluated in six double-blind, actively controlled studies outside the United States that ranged in duration from 4 weeks to 6 months. In these studies, the efficacy of meloxicam at doses of 7.5 and 15 mg/day was comparable to that of piroxicam at 20 mg/day and diclofenac in a prolonged dose of 100 mg/day and comparable to that observed in the US study.
The use of meloxicam to treat signs and symptoms of rheumatoid arthritis was evaluated in a 12-week, double-blind, controlled multinational study. Meloxicam (7.5, 15, and 22.5 mg/d) was compared with placebo. The primary endpoint in this study was the degree of ACR20 response, a composite measure of clinical, laboratory, and functional measures of rheumatoid arthritis response. Patients who received meloxicam at doses of 7.5 and 15 mg daily showed a significant improvement in the primary endpoint compared to placebo.
No additional improvement was observed at the 22.5 mg dose compared with the 15 mg dose. Higher doses of meloxicam (22.5 mg or more) have been associated with an increased risk of serious GI adverse reactions, so the daily dose of meloxicam tablets should not exceed 15 mg.
Carcinogenesis, mutagenesis, impaired fertility
No carcinogenic effects of meloxicam were observed in rats given orally up to 0.8 mg/kg/day (approximately 0.4 dose for a 50 kg adult at 15 mg/day and recalculated per unit body surface area) for 104 weeks, or in mice given orally up to 8 mg/kg/day (approximately 2.2 higher doses for humans, as indicated) for 99 weeks.
Meloxicam showed no mutagenicity in the Ames test or clastogenicity in the human lymphocyte chromosomal abnormality assay and the in vivo micronucleus test in mouse bone marrow.
Meloxicam had no effect on fertility in male and female rats at oral doses of up to 9 and 5 mg/kg/day, respectively (4.9 and 2.5 times higher than for humans, as noted above). However, an increased incidence of fetal death at oral doses of ≥1 mg/kg/day (0.5 dose for humans, as noted above) was observed in rats when female rats received meloxicam 2 weeks before mating and during early embryonic development.
Indications
Indications
Osteoarthritis
rheumatoid arthritis
ankylosing spondylitis (ankylosing spondylitis)
inflammatory and degenerative joint diseases accompanied by pain
Pharmacological effect
Pharmacological effect
Pharmaceutical group:
NSAIDs.
Pharmacology
Mechanism of action
Meloxicam is an NSAID that exhibits anti-inflammatory, analgesic, and antipyretic effects in animal models. The mechanism of action of meloxicam, like other NSAIDs, is associated with COX inhibition.
Like other NSAIDs, meloxicam competitively inhibits both COX isoenzymes – COX-1 and COX-2 – blocking the metabolism of arachidonate, which leads to analgesic, antipyretic and anti-inflammatory pharmacological effects. NSAIDs do not affect the peroxidase reaction and do not suppress the synthesis of leukotrienes via lipoxygenase pathways. Meloxicam has a predominant effect on COX-2.
The gastrointestinal side effects of meloxicam are primarily related to COX-1 inhibition, but the potential role of COX-2 inhibition in the gastrointestinal tract is not fully understood.
In the kidneys, PGs produced with the participation of both COX-1 and COX-2 are important regulators of sodium and water metabolism, as well as renal function and hemodynamics. In conditions where renal blood flow is dependent on the synthesis of PGs, the use of NSAIDs can lead to a significant decrease in renal blood flow, which can cause acute renal failure. In addition, changes in sodium and water reabsorption may worsen the condition of patients with high blood pressure.
Pharmacokinetics
Suction
The absolute bioavailability of meloxicam capsules was 89% after a single oral dose of 30 mg compared with an IV bolus injection of the same dose. After a single intravenous administration, dose-dependent pharmacokinetics were observed in the dose range from 5 to 60 mg. After multiple oral doses, the pharmacokinetics of meloxicam capsules were dose-dependent, ranging from 7.5 to 15 mg. The average Cmax was achieved within 4–5 hours after taking a meloxicam 7.5 mg tablet on an empty stomach, indicating prolonged absorption of the drug. With repeated dosing, stable concentrations were achieved by day 5. A second peak in meloxicam concentrations occurs approximately 12 to 14 hours after dosing, indicating enterohepatic recirculation.
Table 1
Pharmacokinetic parameters of a single dose and equilibrium state during oral administration of meloxicam in doses of 7.5 and 15 mg (mean value and coefficient of variation, %)
Pharmacokinetic parameter1
State of balance
Single dose
Healthy adult men, 7.5 mg tablets, after a low-fat meal (n=18)
Elderly men, 15 mg capsules, after a low-fat meal (n=5)
Elderly women, 15 mg capsules, after a low-fat meal (n=8)
Renal failure, 15 mg capsules, on an empty stomach (n=12)
Liver failure, 15 mg capsules, fasting (n=12)
Cmax, µg/ml
1.05 (20)
2.3 (59)
3.2 (24)
0.59 (36)
0.84 (29)
Tmax, h
4.9 (8)
5 (12)
6 (27)
4 (65)
10 (87)
T1/2, h
20.1 (29)
21 (34)
24 (34)
18 (46)
16 (29)
CL/f, ml/min
8.8 (29)
9.9 (76)
5.1 (22)
19 (43)
11 (44)
Vz/f (dose/(AUC·Kel), l
14.7 (32)
15 (42)
10 (30)
26 (44)
14 (29)
1 Parameter values in the table are taken from various studies.
Eating food and antacids. Administration of meloxicam capsules after a high-fat breakfast (75 g fat) resulted in an approximately 22% increase in Cmax, while AUC was unchanged. Tmax ranged from 5 to 6 hours. PEF was not detected with simultaneous use of antacids. Based on these data, meloxicam tablets can be prescribed regardless of the time of meals or the simultaneous use of antacids.
Distribution
The average Vss of meloxicam is approximately 10 L. Meloxicam is approximately 99.4% bound to plasma proteins (primarily albumin) within the therapeutic dose range. Protein binding is independent of meloxicam concentrations over a clinically relevant range, but decreases to approximately 99% in patients with renal impairment. The penetration of meloxicam into human red blood cells after oral administration is less than 10%. After administration of a radiolabeled dose, more than 90% of the radioactivity detected in plasma was present as unchanged meloxicam.
The concentration of meloxicam in synovial fluid after a single oral dose ranges from 40 to 50% of the plasma concentration. The free fraction in synovial fluid is 2.5 times higher than in plasma due to the lower albumin content in synovial fluid compared to plasma. The significance of this phenomenon is unknown.
Metabolism
Meloxicam is almost completely metabolized to four pharmacologically inactive metabolites. The main metabolite, 5′-carboxymeloxicam (60% of the dose), is formed as a result of the oxidation of the intermediate metabolite 5′-hydroxymethylmeloxicam, the degree of elimination of which is 9% of the dose. In vitro studies indicate that CYP2C9 plays an important role in this metabolic pathway, with a minor contribution from the CYP3A4 isoenzyme. Peroxidase activity is likely responsible for two other metabolites, which account for 16 and 4% of the administered dose, respectively.
Removal
Meloxicam is excreted primarily in the form of metabolites and equally in urine and feces. Only traces of the unchanged parent compound are excreted in urine (0.2%) and feces (1.6%). The extent of urinary excretion was confirmed for unlabeled multiple doses of 7.5 mg – 0.5, 6 and 13% of the dose were detected in urine as meloxicam, 5′-hydroxymethyl- and 5′-carboxymetabolites, respectively. Significant biliary and/or enteral secretion of meloxicam is observed.
This was demonstrated when oral administration of cholestyramine following a single IV dose of meloxicam reduced its AUC by 50%. The average T1/2 is from 15 to 20 hours. T1/2 does not change at different dose levels, indicating linear metabolism over the range of therapeutic doses. Plasma clearance ranges from 7 to 9 ml/min.
Special patient groups
Old age. In elderly men (≥65 years), meloxicam plasma concentrations and pharmacokinetics at steady state were similar to those observed in younger patients. In older women (≥65 years), after normalization of body weight, AUCss was 47% and Cmax, ss were 32% higher than in younger women (≤55 years). Despite this increase, the adverse event profile was comparable for both groups of patients. A lower free fraction was found in elderly female patients compared to elderly male patients.
Floor. In young women, plasma concentrations are slightly lower than in young men. After a single dose of 7.5 mg meloxicam, the mean T1/2 was 19.5 hours for women compared to 23.4 hours for men. In the equilibrium state, the data were similar (17.9 vs. 21.4 hours). This gender difference is probably of little clinical significance. Linearity of pharmacokinetics was observed and there were no significant differences in Cmax or Tmax values between men and women.
Liver failure. After a single dose of meloxicam 15 mg, no significant differences in plasma concentrations were observed in patients with mild (Child-Pugh class A) and moderate (Child-Pugh class B) hepatic impairment compared with healthy volunteers. Liver failure did not affect the binding of meloxicam to plasma proteins. For mild to moderate liver failure, no dose adjustment is required. Sufficient studies have not been conducted in patients with severe hepatic impairment (Child-Pugh class C).
Kidney failure. The pharmacokinetics of meloxicam have been studied in patients with varying degrees of renal insufficiency. The total drug concentration in plasma decreased depending on the severity of renal failure, while the AUC value of the free fraction was similar. The total clearance of meloxicam was increased in patients with renal impairment, probably due to an increase in the free fraction, which resulted in accelerated metabolic clearance.
In patients with mild to moderate renal failure (creatinine clearance >15 ml/min), no dose adjustment is required. Patients with severe renal failure have not been well studied. The use of meloxicam in patients with severe renal impairment is not recommended.
Hemodialysis. After a single dose of meloxicam, the Cmax of the free fraction in plasma was higher in patients with renal failure on chronic hemodialysis (free fraction 1%) compared with healthy volunteers (0.3%). Hemodialysis did not reduce the total plasma concentration of meloxicam. Thus, additional doses after hemodialysis are not required. Meloxicam is not dialyzable.
Clinical studies
Osteoarthritis and rheumatoid arthritis. Meloxicam for the treatment of signs and symptoms of osteoarthritis of the knee and hip was evaluated in a 12-week, double-blind, controlled study. Meloxicam (3.75, 7.5 and 15 mg/day) was compared with placebo. The four primary endpoints were investigator global assessment, patient global assessment, patient pain assessment, and WOMAC (self-administered pain, function, and stiffness) total score. Patients receiving meloxicam 7.5 and 15 mg/day showed significant improvement in each of these endpoints compared with placebo.
The use of meloxicam for the treatment of signs and symptoms of osteoarthritis was evaluated in six double-blind, actively controlled studies outside the United States, the duration of which ranged from 4 weeks to 6 months. In these studies, the effectiveness of meloxicam at doses of 7.5 and 15 mg/day was comparable to the use of piroxicam at a dose of 20 mg/day and diclofenac extended-release at a dose of 100 mg/day and comparable to the effectiveness observed in the US study.
The use of meloxicam for the treatment of signs and symptoms of rheumatoid arthritis was evaluated in a 12-week, double-blind, controlled, multinational study. Meloxicam (7.5, 15 and 22.5 mg/day) was compared with placebo. The primary endpoint of this study was ACR20 response rate, a composite measure of clinical, laboratory, and functional measures of response in rheumatoid arthritis. Patients receiving meloxicam 7.5 and 15 mg daily experienced significant improvement in the primary endpoint compared with placebo.
No additional improvement was observed with the 22.5 mg dose compared to the 15 mg dose. Higher doses of meloxicam (22.5 mg or more) have been associated with an increased risk of serious gastrointestinal adverse reactions, so the daily dose of meloxicam tablets should not exceed 15 mg.
Carcinogenesis, mutagenesis, impaired fertility
No carcinogenic effect of meloxicam was observed in rats administered orally up to 0.8 mg/kg/day (approximately 0.4 times the dose for a 50-kg adult at 15 mg/day per body surface area) for 104 weeks, or in mice treated orally up to 8 mg/kg/day (approximately 2.2 times the human dose as above) for 104 weeks. 99 weeks
Meloxicam was not mutagenic in the Ames test or clastogenic in the human lymphocyte chromosomal abnormality assay and the in vivo micronucleus test in mouse bone marrow.
Meloxicam did not affect the fertility of male and female rats at oral doses of up to 9 and 5 mg/kg/day, respectively (4.9 and 2.5 times higher than for humans, as noted above). However, an increased incidence of fetal death at oral doses ≥1 mg/kg/day (0.5 human dose, as noted above) was observed in rats when females received meloxicam 2 weeks before mating and during early embryonic development in amounts (0.2% in urine and 1.6% in feces).
Special instructions
Special instructions
Meloxicam is intended for symptomatic therapy and should not be taken to prevent exacerbation of diseases. Caution should be exercised when treating patients with a history of gastrointestinal diseases and in patients receiving anticoagulants.
Patients experiencing gastrointestinal symptoms should be monitored regularly. Periodic monitoring of blood clotting parameters is necessary.
If ulcerative lesions of the gastrointestinal tract or gastrointestinal bleeding occur, meloxicam should be discontinued. If allergic reactions (itching, urticaria, skin rash, photosensitivity) occur during treatment, you should consult a doctor to decide whether to stop taking the drug.
The use of NSAIDs in patients with reduced renal blood flow or reduced circulating blood volume may lead to decompensation of latent renal failure. After discontinuation of NSAIDs, renal function usually returns to baseline levels.
Elderly patients are most at risk of developing this reaction; patients who experience dehydration; patients with congestive heart failure, liver cirrhosis, nephrotic syndrome or kidney disease; patients receiving diuretics, ACE inhibitors, angiotensin II receptor antagonists; as well as patients who have undergone major surgical procedures leading to hypovolemia.
In such patients, diuresis and renal function should be carefully monitored when initiating therapy.
The increase in transaminase activity is usually transient and insignificant. If there is a significant and persistent increase in transaminase activity, meloxicam should be discontinued and liver function monitored. Weakened or malnourished patients may be less able to tolerate adverse events and such patients should be monitored closely.
Caution should be used when treating elderly patients, who are more likely to have impaired renal, hepatic, and cardiac function.
The use of NSAIDs in combination with diuretics can lead to sodium, potassium and water retention, and affect the natriuretic effect of diuretics. As a result, predisposed patients may experience increased signs of heart failure or hypertension.
Meloxicam, like other NSAIDs, can mask the symptoms of an infectious disease. The use of meloxicam, like other drugs that block prostaglandin synthesis, can affect fertility, so the drug is not recommended for women wishing to become pregnant.
Impact on the ability to drive vehicles and operate machinery
No special studies have been conducted regarding the effect of the drug on the ability to drive vehicles and operate machinery.
You should drive with caution and engage in other potentially hazardous activities that require a high speed of psychomotor reactions, due to possible adverse reactions from the central nervous system, such as visual disturbances, drowsiness, and dizziness.
Active ingredient
Active ingredient
Meloxicam
Composition
Composition
1 tablet contains
Active substance – meloxicam 15.0 mg;
Excipients:
Microcrystalline cellulose 30.0 mg,
Lactose monohydrate 140.0 mg,
Colloidal silicon dioxide (Aerosil) – 3.0 mg,
Croscarmellose sodium (primellose) – 6.0 mg,
Talc 3.0 mg,
Magnesium stearate 3.0 mg.
Pregnancy
Pregnancy
Meloxicam is contraindicated in children under 15 years of age, during pregnancy and breastfeeding.
Contraindications
Contraindications
Hypersensitivity to meloxicam and/or to any of the components of the drug, a combination of bronchial asthma and recurrent polyposis of the nose and paranasal sinuses, intolerance to acetylsalicylic acid and pyrazolone drugs;
peptic ulcer of the stomach and duodenum in the acute phase, active gastrointestinal bleeding, other bleeding; severe liver failure and active liver disease; severe renal failure (if hemodialysis is not performed and creatinine clearance is less than 30 ml/min),
progressive kidney disease, hyperkalemia; severe heart failure, condition after coronary artery bypass grafting; pregnancy, lactation (breastfeeding); children under 12 years of age, lactase deficiency, galactosemia, glucose-galactose malabsorption syndrome.
With caution
Age over 65 years, coronary heart disease, chronic heart failure, cerebrovascular diseases, dyslipidemia, hyperlipidemia, diabetes mellitus, diseases
peripheral arteries, smoking, creatinine clearance less than 60 ml/min, a history of ulcerative lesions of the gastrointestinal tract, the presence of Helicobacter púlori infection, long-term use of NSAIDs, alcoholism, severe somatic
diseases, simultaneous use of oral glucocorticosteroids (anticoagulants (including warfarin), antiplatelet agents (including clopidogrel), selective serotonin reuptake inhibitors
(including citalopram, fluoxetine, paroxetine, sertraline), angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists.
Side Effects
Side Effects
From the digestive system: dyspepsia, incl. nausea, vomiting, abdominal pain, diarrhea, constipation, flatulence, belching; erosive and ulcerative lesions of the gastrointestinal tract, perforation of the stomach or intestines, gastrointestinal bleeding (latent or obvious); transient increase in the activity of liver transaminases, hyperbilirubinemia, hepatitis, esophagitis, stomatitis, gastritis, colitis, dry mouth.
From the cardiovascular system: peripheral edema, tachyardia, increased or decreased blood pressure, hot flashes, vasculitis.
From the hematopoietic organs: anemia, leukopenia, thrombocytopenia.
From the respiratory system: bronchospasm, bronchial asthma with aspirin intolerance (unstable bronchial asthma).
From the central nervous system: dizziness, headache, tinnitus, sleep disturbances (drowsiness/insomnia), disorientation, emotional lability, confusion, anxiety, depression.
From the urinary system: hematuria, albuminuria, increased creatinine levels, acute renal failure.
Allergic reactions: anaphylactoid and anaphylactic reactions, itching, skin rash, urticaria, erythema multiforme, Steven-Johnson syndrome, toxic epidermal necrolysis (Lyell’s syndrome), photosensitivity.
Other: disturbance of taste, conjunctivitis, visual disturbances (blurredness), weakness, fever.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
If you notice any allergic reactions to any drug, such as rash, itching, difficulty swallowing and breathing, swelling of the larynx and tongue, stop taking the drug immediately and consult a doctor.
Interaction
Interaction
When used simultaneously with acetylsalicylic acid and other NSAIDs, the risk of erosive and ulcerative lesions and bleeding from the gastrointestinal tract increases.
When used simultaneously with antihypertensive drugs, the effectiveness of the latter may be reduced.
When used simultaneously with lithium preparations, accumulation of lithium and an increase in its toxic effect are possible (monitoring the concentration of lithium in the blood is recommended).
Simultaneous use with methotrexate increases the side effects of the latter on the hematopoietic system (there is a risk of developing anemia and leukopenia.
Periodic monitoring of a general blood test is necessary). Combined use with diuretics and cyclosporine leads to an increased risk of developing renal failure.
When used simultaneously with intrauterine contraceptives, the effectiveness of the latter may decrease.
When used simultaneously with anticoagulants (heparin, ticlopidine, warfarin), as well as with thrombolytic drugs (streptokinase, fibrinolysin), the risk of bleeding increases (blood clotting indicators should be periodically monitored).
Simultaneous use with cholestyramine increases the excretion of meloxicam through the gastrointestinal tract (as a result of binding of meloxicam). No pharmacokinetic interaction was detected when meloxicam was taken concomitantly with antacids.
Overdose
Overdose
Symptoms: increased side effects.
Treatment: gastric lavage, taking activated carbon (within the next hour), symptomatic therapy.
Cholestyramine accelerates the elimination of the drug from the body. Forced diuresis, alkalization of urine, hemodialysis are ineffective due to the high binding of meloxicam to blood proteins. No specific antidotes or antagonists have been found.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years. Do not use the drug after the expiration date indicated on the package.
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 3 years. Do not use the drug after the expiration date stated on the package. |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Vertex, Russia |
| Medication form | pills |
| Brand | Vertex |
Other forms…
Related products
Buy Meloxicam-Vertex, tablets 15 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.