No products in the cart.
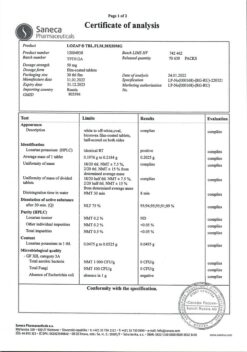
Lozap, 100 mg 60 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
Hypertension (high blood pressure), Prevention of heart attacks and strokes
- Arterial hypertension;
- Cronic heart failure (as part of combined therapy, with intolerance or inefficiency of ACE inhibitor therapy);
- Risk reduction for cardiovascular disease (including stroke) and mortality in patients with arterial hypertension and left ventricular hypertrophy;
- Diabetic nephropathy with hypercreatininemia and proteinuria (urine albumin to creatinine ratio greater than 300 mg/g) in patients with type 2 diabetes and concomitant arterial hypertension (reducing progression of diabetic nephropathy to terminal chronic renal failure).
.
Active ingredient
Active ingredient
Composition
Composition
coated film-coated tablets.
1 film-coated tablet contains:
Active substance
Potassium losartan 100 mg
Associates
Core
Microcrystalline cellulose,
mannitol, crosspovidone,
p> colloidal anhydrous silica,
talc,
magnesium stearate.
Film coating
. Sepiphilm 752 (white) (hypromellose, microcrystalline cellulose, macrogoal 2000 stearate, titanium dioxide),
macrogoal 6000.
How to take, the dosage
How to take, the dosage
Lozap® is taken orally, regardless of meals, and the frequency of administration is once a day.
In case of arterial hypertension, the average daily dose is 50 mg. In individual cases, to achieve a greater therapeutic effect, the dose is increased to 100 mg in two doses or once a day. Starting dose for patients with chronic heart failure is 12.5 mg 1 time per day (1 tablet of LOSAP® 12.5 mg). As a rule, the dose is increased with weekly intervals (i.e. 12.5 mg/day, 25 mg/day and 50 mg/day) up to an average maintenance dose of 50 mg once daily, depending on patient’s tolerability.
When prescribing the drug in patients receiving high-dose diuretics the initial dose of LOSAP® should be decreased to 25 mg (? LOSAP® 50 mg tablets) once daily.
It is not necessary to adjust the dose in elderly patients.
Limiting the risk of cardiovascular diseases (including stroke) and mortality in patients with arterial hypertension and left ventricular hypertrophy: the initial dose of the drug is 50 mg once daily.
Lower doses of hydrochlorothiazide may be added and/or the dose of LOSAP® may be increased up to 100 mg daily in one or two doses.
In patients with concomitant diabetes mellitus type 2 with proteinuria: LOSAP® is prescribed in initial dose – 50 mg once a day with further increasing of dose up to 100 mg/day (taking into account the degree of blood pressure decrease) in one or two doses.
Patients with a history of liver diseases, dehydration, hemodialysis procedure and patients older than 75 years old should be prescribed lower initial dose of the preparation – 25 mg (? LOSAP® 50 mg tablets) once daily.
Interaction
Interaction
Can be administered with other hypotensive agents. Mutually enhances the effect of beta-adrenoblockers and sympatholytics.
The co-administration of losartan with diuretics has an additive effect. No pharmacokinetic interactions of losartan with hydrochlorothiazide, digoxin, warfarin, cimetidine, phenobarbital, ketoconazole and erythromycin have been noted.
Rifampicin and fluconazole have been reported to decrease plasma levels of the active metabolite. The clinical significance of these interactions is not yet known.
As with other agents that inhibit angiotensin II or its effects, co-administration of losartan with potassium-saving diuretics (e.g., spironolactone, triamterene, amiloride), potassium preparations, and salts containing potassium increases the risk of hyperkalemia. Non-steroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors, may reduce the effect of diuretics and other hypotensive drugs.
The co-administration of angiotensin II receptor antagonists and lithium may increase plasma lithium concentrations. Given this, the benefit and risk of co-administration of losartan with lithium salts should be weighed.
If the drugs are to be used together, plasma lithium concentrations should be monitored regularly.
Special Instructions
Special Instructions
Correction of dehydration should be performed before prescribing LOSAP® or treatment should be started with a lower dose of the drug.
The drugs affecting the renin-angiotensin system may increase blood urea and serum creatinine concentrations in patients with bilateral renal stenosis or arterial stenosis of the sole kidney.
In patients with cirrhosis, plasma concentrations of losartan are significantly increased, so it should be prescribed at lower doses if there is a history of liver disease.
Potassium concentration in blood should be monitored regularly during treatment, especially in elderly patients with renal dysfunction.
Effect on ability to concentrate:
lozartan does not affect the ability to drive or operate machinery.
Contraindications
Contraindications
With caution: arterial hypotension, decreased volume of circulating blood, electrolyte-water balance disorders, bilateral renal artery stenosis or stenosis of the artery of the single kidney, renal/hepatic insufficiency.
Overdose
Overdose
Symptoms: marked BP decrease, tachycardia, due to parasympathetic (vagus) stimulation bradycardia may develop.
Treatment: forced diuresis, symptomatic therapy; hemodialysis is not effective.
Similarities
Similarities
Additional information
| Manufacturer | Saneka Pharmaceuticals a.s., Slovakia |
|---|---|
| Medication form | pills |
| Brand | Saneka Pharmaceuticals a.s. |
Other forms…
Related products
Buy Lozap, 100 mg 60 pcs. with delivery to USA, UK, Europe and over 120 other countries.