No products in the cart.
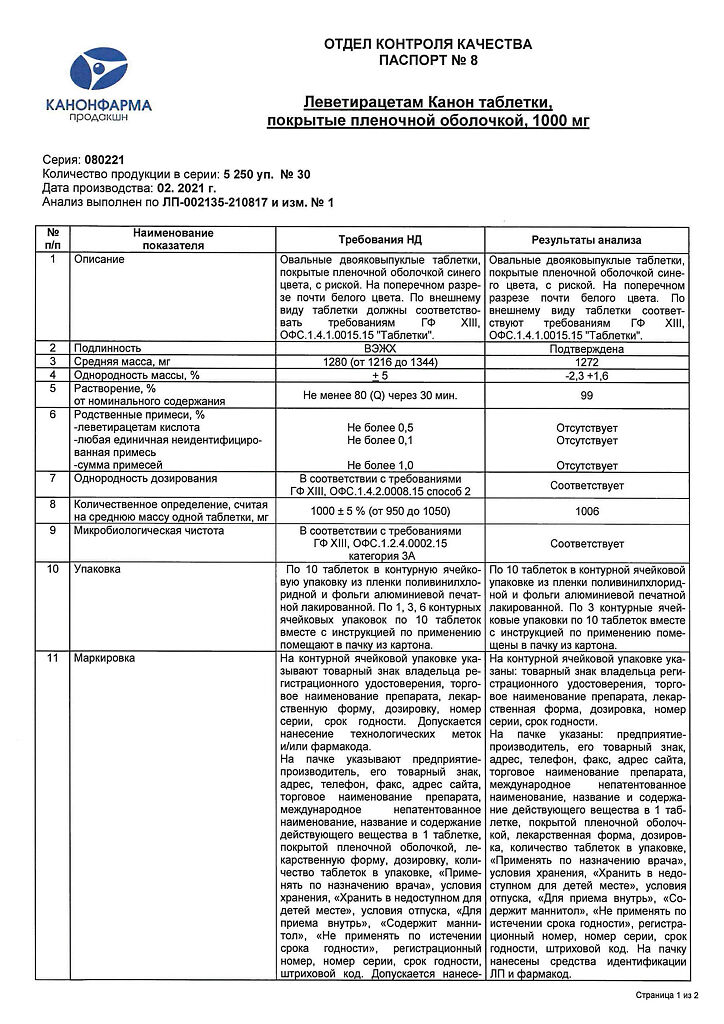
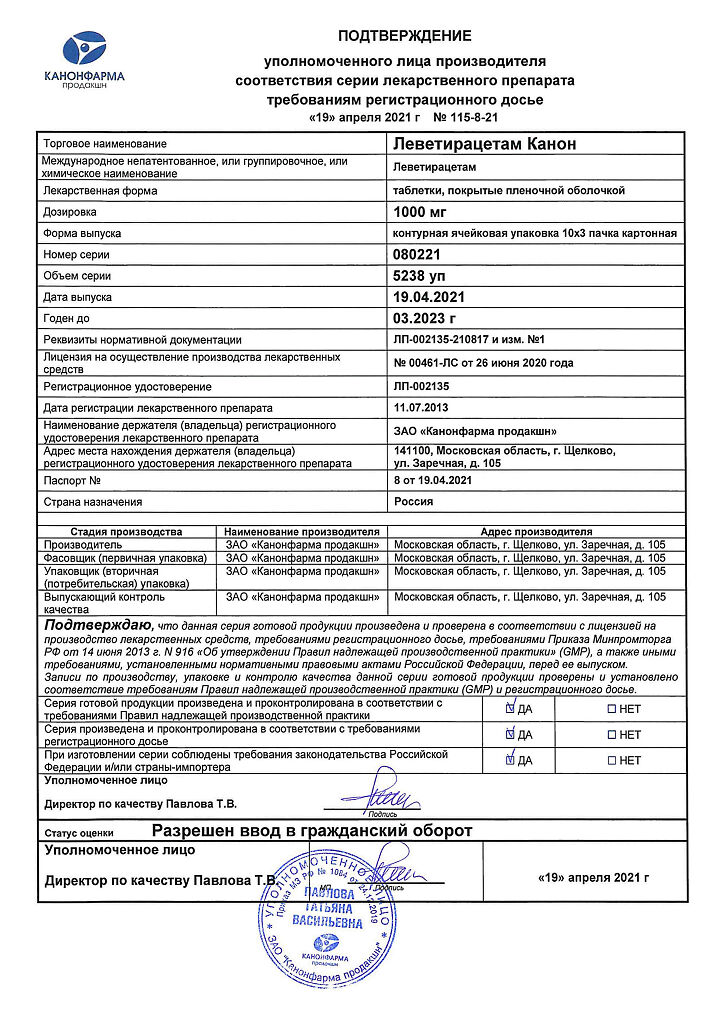
Levetiracetam Canon, 1000 mg 30 pcs
€25.33 €21.11
Description
Pharmacodynamics
Levetiracetam is a pyrrolidone derivative (S-enantiomer of α-ethyl-2-oxo-1-pyrrolidine acetamide) and differs from known antiepileptic drugs by chemical structure. The mechanism of action of levetiracetam is not fully understood, but it is obvious that it differs from the mechanism of action of known antiepileptic drugs.
In vitro and in vivo experiments have shown that levetiracetam does not affect basic cell characteristics and normal transmission. In in vitro studies it has been shown to affect intraneuronal Ca2+ ion concentration by partially inhibiting Ca2+ ion current through N-type channels and reducing calcium release from intraneuronal depots.
In addition, it partially restores currents through GABA- and glycine-dependent channels reduced by zinc and carbolines. One putative mechanism is based on the proven binding to the synaptic glycoprotein SV2A vesicles contained in the gray matter of the brain and spinal cord. It is believed that this is the way the anticonvulsant effect is realized, which is expressed in the counteraction of hypersynchronization of neuronal activity.
Levetiracetam also acts on GABA receptors and glycine receptors, modulating these receptors through various endogenous agents. The drug does not alter normal neurotransmission, but suppresses epileptiform neuronal bursts induced by the GABA agonist bicuculline and excitation of glutamate receptors.
The activity of the drug has been confirmed against both focal and generalized epileptic seizures (epileptiform manifestations/photoparoxysmal response).
Pharmacokinetics
No dependence of pharmacokinetics on sex, race and time of day was observed.
Intake.
Levetiracetam is well absorbed from the gastrointestinal tract after oral administration. Levetiracetam is well soluble in water and has high permeability.
The absorption is complete and linear, so that plasma concentrations can be predicted based on the dose of levetiracetam taken, expressed in mg/kg body weight. The degree of absorption is independent of the dose and time of ingestion.
The bioavailability is approximately 100%. The maximum plasma concentration (Cmax) is reached 1.3 hours after oral administration of levetiracetam in a dose of 1000 mg and is 31 µg/ml in a single dose, after repeated administration (twice daily) – 43 µg/ml. The equilibrium state is reached after 2 days with twice-daily administration of the drug.
Distribution.
The binding of levetiracetam and its main metabolite to plasma proteins is less than 10%. The volume of distribution (Vd) is approximately 0.5-0.7 L/kg, which is approximately the volume of water in the body.
There are no data on the distribution of the drug in tissues.
Metabolism.
Levetiracetam is poorly metabolized in the human body.
The major metabolic pathway (24% of the dose taken) is enzymatic hydrolysis of the acetamide group. The formation of the primary metabolite (ucb L057) occurs without the participation of hepatic cytochrome P450. The metabolite ucb L057 is pharmacologically inactive. Levetiracetam does not affect the enzymatic activity of hepatocytes.
Under in vitro conditions, levetiracetam and its major metabolite did not inhibit major forms of cytochrome P450 (CYP3A4, 2A6, 2C9, 2C19, 2D6, 2E1, 1A2) or glucuronyl transferase (UGT1A1, UGT1A6) and epoxide hydroxylase activity. Did not affect glucuronidation of valproic acid in vitro.
Elimination.
The elimination half-life (T1/2) from adult blood plasma is 7±1 h and is independent of the route of administration and dosing regimen. Mean value of the total clearance is 0.96 ml/min/kg. 95% of the drug is excreted by the kidneys. Renal clearance of levetiracetam and its metabolite is 0.6 and 4.2 ml/min/kg, respectively.
Pharmacokinetics in special clinical cases
Elderly patients
The T1/2 in elderly patients is increased by 40% and is 10-11 h, which is associated with impaired renal function in this category of people.
Renal impairment
In patients with impaired renal function, the clearance of levetiracetam and its primary metabolite correlates with creatinine clearance. Therefore, in patients with renal impairment, it is recommended that the dose be adjusted according to creatinine clearance (see section “Dosage and administration”).
In end-stage renal failure in adult patients the T1/2 is 25 h between dialysis sessions and 3.1 h during dialysis. Up to 51% of levetiracetam is removed during a 4-hour dialysis session.
Hepatic impairment
In patients with mild to moderate hepatic impairment, there are no significant changes in the clearance of levetiracetam. In most patients with severe hepatic impairment with concomitant renal impairment, levetiracetam clearance is reduced by more than 50%.
Children aged 4-12 years
After a single dose of 20 mg/kg, the T1/2 in children aged 4-12 years is 6 hours. Total clearance of levetiracetam in children 4-12 years old is approximately 30% higher and is in direct correlation with body weight.
After repeated administration of the drug at a dose of 20-60 mg/kg body weight in children 4-12 years of age, the maximum plasma concentration is reached after 0.5-1.0 hours and increases linearly and in proportion to the dose.
Indications
Indications
As monotherapy (drug of first choice) in the treatment
partial seizures with or without secondary generalization in adults and adolescents over 16 years of age with newly diagnosed epilepsy.
As part of complex therapy for the treatment
partial seizures with or without secondary generalization in adults and children over 6 years of age suffering from epilepsy;
myoclonic seizures in adults and adolescents over 12 years of age suffering from juvenile myoclonic epilepsy;
primary generalized convulsive (tonic-clonic) seizures in adults and adolescents over 12 years of age suffering from idiopathic generalized epilepsy.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Levetiracetam is a derivative of pyrrolidone (S-enantiomer of α-ethyl-2-oxo-1-pyrrolidine acetamide), whose chemical structure differs from known antiepileptic drugs. The mechanism of action of levetiracetam is not fully understood, but it is obvious that it differs from the mechanism of action of known antiepileptic drugs.
In vitro and in vivo experiments have shown that levetiracetam does not affect basic cell characteristics and normal transmission. In vitro studies have shown that it affects the intraneuronal concentration of Ca2+ ions, partially inhibiting the current of Ca2+ ions through N-type channels and reducing the release of calcium from intraneuronal stores.
In addition, it partially restores currents through GABA- and glycine-dependent channels reduced by zinc and carbolines. One of the proposed mechanisms is based on proven binding to the synaptic vesicle glycoprotein SV2A, contained in the gray matter of the brain and spinal cord. It is believed that in this way an anticonvulsant effect is realized, which is expressed in counteracting the hypersynchronization of neural activity.
Levetiracetam also acts on GABA receptors and glycine receptors, modulating these receptors through various endogenous agents. The drug does not alter normal neurotransmission, but suppresses epileptiform neuronal bursts induced by the GABA agonist bicuculline and the excitation of glutamate receptors.
The activity of the drug has been confirmed against both focal and generalized epileptic seizures (epileptiform manifestations/photoparoxysmal reaction).
Pharmacokinetics
There was no dependence of pharmacokinetics on gender, race or time of day.
Suction.
After oral administration, levetiracetam is well absorbed from the gastrointestinal tract. Levetiracetam is highly soluble in water and has high penetrating ability.
Absorption is complete and linear, so plasma concentrations can be predicted based on the dose of levetiracetam taken, expressed in mg/kg body weight. The degree of absorption does not depend on the dose and time of food intake.
Bioavailability is approximately 100%. The maximum plasma concentration (Cmax) is achieved 1.3 hours after oral administration of levetiracetam at a dose of 1000 mg and is 31 mcg/ml after a single dose, and 43 mcg/ml after repeated doses (2 times a day). An equilibrium state is achieved after 2 days with a double dose of the drug.
Distribution.
The binding of levetiracetam and its main metabolite to plasma proteins is less than 10%. The volume of distribution (Vd) is approximately 0.5-0.7 l/kg, which approximately corresponds to the volume of water in the body.
There are no data on the distribution of the drug across tissues.
Metabolism.
Levetiracetam is poorly metabolized in the human body.
The main route of metabolism (24% of the dose taken) is enzymatic hydrolysis of the acetamide group. The formation of the primary metabolite (ucb L057) occurs without the participation of liver cytochrome P450. Metabolite ucb L057 is pharmacologically inactive. Levetiracetam does not affect the enzymatic activity of hepatocytes.
In vitro, levetiracetam and its main metabolite did not inhibit the main forms of cytochrome P450 (CYP3A4, 2A6, 2C9, 2C19, 2D6, 2E1, 1A2), as well as the activity of glucuronyl transferase (UGT1A1, UGT1A6) and epoxide hydroxylase. Did not affect the glucuronidation of valproic acid in vitro.
Excretion.
The half-life (T1/2) from the blood plasma of an adult is 7±1 hours and does not depend on the route of administration and dosage regimen. The average total clearance is 0.96 ml/min/kg. 95% of the drug is excreted by the kidneys. The renal clearance of levetiracetam and its metabolite is 0.6 and 4.2 ml/min/kg, respectively.
Pharmacokinetics in special clinical situations
Elderly patients
In elderly patients, T1/2 increases by 40% and is 10-11 hours, which is associated with impaired renal function in this category of people.
Kidney failure
In patients with impaired renal function, the clearance of levetiracetam and its primary metabolite correlates with creatinine clearance. Therefore, in patients with renal failure, it is recommended that the dose be adjusted depending on creatinine clearance (see section “Dosage and Administration”).
In end-stage renal failure in adult patients, T1/2 is 25 hours between dialysis sessions and 3.1 hours during dialysis. During a 4-hour dialysis session, up to 51% of levetiracetam is removed.
Liver dysfunction
In patients with mild to moderate hepatic impairment, there are no significant changes in the clearance of levetiracetam. In most patients with severely impaired liver function and concomitant renal failure, the clearance of levetiracetam is reduced by more than 50%.
Children aged 4-12 years
After a single dose of the drug at a dose of 20 mg/kg, T1/2 in children 4-12 years old is 6 hours. The total clearance of levetiracetam in children 4-12 years old is approximately 30% higher and is directly dependent on body weight.
After repeated administration of the drug at a dose of 20-60 mg/kg body weight to children 4-12 years old, the maximum plasma concentration is reached after 0.5-1.0 hours and increases linearly and in proportion to the dose.
Special instructions
Special instructions
Cancellation of therapy
It is recommended to discontinue the drug gradually. For example, in adults and adolescents weighing more than 50 kg, the dose should be reduced in increments of 500 mg 2 times a day no more often than every 2-4 weeks; in children, the dose should be reduced in increments of no more than 10 mg/kg 2 times a day no more often than every two weeks.
Concomitant antiepileptic drugs (during the transfer of patients to levetiracetam therapy) should preferably be discontinued gradually.
Kidney failure
The use of levetiracetam in patients with renal failure may require dose adjustment. In patients with severe hepatic impairment, it is recommended to evaluate renal function before starting dose selection (see Dosage and Administration).
Suicide
Suicide, suicide attempts, and suicidal thoughts and behavior have been reported in patients taking anticonvulsants (including levetiracetam). Because of the above, patients with symptoms of depression or suicidal thoughts and behavior should be monitored and treated accordingly.
Patients (and their caregivers) should be advised to seek medical attention if they experience symptoms of depression and/or suicidal thoughts and behavior.
Children
The tablets are not intended for use in children under 6 years of age. For children under 6 years of age, the recommended dosage form is oral solution.
According to available data, levetiracetam does not affect growth and puberty. However, long-term effects on learning, intelligence, growth, endocrine function, puberty, and fertility in children are not known.
Impact on the ability to drive vehicles and machinery
The effect of levetiracetam on the ability to drive vehicles and operate machinery has not been specifically studied.
However, due to varying individual sensitivity to the drug, some patients may experience drowsiness and other central nervous system disturbances, especially at the beginning of therapy and after dose increases.
Therefore, it is recommended to refrain from driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Levetiracetam
Composition
Composition
1 film-coated tablet contains:
active ingredient:
levetiracetam 1000.0 mg;
excipients:
calcium stearate 12.0 mg,
colloidal silicon dioxide 12.0 mg,
croscarmellose sodium 40.0 mg,
mannitol 80.8 mg,
povidone K-30 25.2 mg,
microcrystalline cellulose (type 101) 70 mg;
composition of the film shell: Opadry II 85F205008 blue 40.0 mg, including: polyvinyl alcohol 16.00 mg, macrogol (polyethylene glycol) 8.08 mg, talc 5.92 mg, titanium dioxide 8.20 mg, indigo carmine dye 1.72 mg, charming red dye 0.08 mg.
Contraindications
Contraindications
Hypersensitivity to levetiracetam or other pyrrolidone derivatives, as well as to any components of the drug.
With caution:
elderly patients (over 65 years old);
liver diseases in the stage of decompensation;
renal failure (creatinine clearance less than 50 ml/min).
Side Effects
Side Effects
Security Profile Summary
The adverse event profile presented below is based on the results of an analysis of pooled placebo-controlled clinical trials of levetiracetam for all indications, as well as post-marketing data.
The most commonly reported adverse reactions were nasopharyngitis, drowsiness, headache, fatigue and dizziness. The safety profile of levetiracetam generally does not differ depending on age (adults and children).
WHO classification of the incidence of side effects:
very often – ≥1/10 prescriptions (>10%)
often – from ≥1/100 to 1% and <10%)
uncommon – from ≥1/1000 to 0.1% and <1%)
rarely – from ≥1/10000 to 0.01% and <0.1%)
very rare – <1/10,000 prescriptions (<0.01%)
Infectious and parasitic diseases
Very common: nasopharyngitis.
Rarely: infections.
Blood and lymphatic system disorders
Uncommon: thrombocytopenia, leukopenia.
Rarely: pancytopenia, agranulocytosis, neutropenia.
Immune system disorders
Rare: drug reaction with eosinophilia and systemic manifestations (DRESS).
Metabolic and nutritional disorders
Common: anorexia.
Uncommon: weight loss or gain.
Rarely: hyponatremia.
Mental disorders
Common: depression, hostility/aggressiveness, insomnia, nervousness, irritability.
Uncommon: suicide attempts, suicidal ideation, psychotic disorders, behavioral disorders, hallucinations, anger, confusion, emotional lability/mood changes, agitation, panic attacks.
Rare: completed suicide, personality disorder, thought disorder.
Nervous system disorders
Very common: drowsiness, headache.
Common: convulsions, dizziness, tremors, imbalance, lethargy.
Uncommon: incoordination or ataxia, amnesia, attention disorder, memory impairment, paresthesia.
Rarely: choreoathetosis, dyskinesia, hyperkinesia.
Visual disorders
Uncommon: diplopia, impaired accommodation.
Hearing and labyrinth disorders
Common: vertigo.
Respiratory system disorders
Often: cough.
Gastrointestinal disorders
Common: abdominal pain, diarrhea, dyspepsia, nausea, vomiting.
Rarely: pancreatitis.
Disorders of the liver and biliary tract
Uncommon: changes in liver function tests.
Rarely: liver failure, hepatitis.
Skin and subcutaneous tissue disorders
Common: skin rash.
Uncommon: eczema, itching, alopecia.
Rare: toxic epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme.
Musculoskeletal and connective tissue disorders
Uncommon: muscle weakness, myalgia.
Common disorders:
Common: asthenia/fatigue
Injuries, complications of procedures:
Uncommon: accidental damage
Description of selected adverse reactions
With simultaneous use of topiramate and levetiracetam, the risk of developing anorexia increases.
In some cases, alopecia has reversed after discontinuation of levetiracetam.
Bone marrow suppression has been detected in some patients with pancytopenia.
Children
The adverse event profile of levetiracetam generally does not differ depending on age (adults and children), and does not depend on the approved indication for use (epilepsy).
With the exception of behavioral and psychiatric adverse reactions, which occurred more frequently in children than in adults, the safety profile of levetiracetam in children was comparable to that in adults in placebo-controlled studies.
In children aged 4-16 years, vomiting (very common, 11.2%), agitation (common, 3.4%), mood changes (common, 2.1%), emotional lability (common, 1.7%), aggression (common, 8.2%), behavioral disturbances (common, 5.6%) and lethargy (common, 3.9%) were observed more often than in other age groups and the overall safety profile. In children aged 1 month to 4 years, irritability (very common, 11.7%) and incoordination (common, 3.3%) were reported more often than in other age groups and the overall safety profile.
The cognitive and neuropsychological effects of levetiracetam in children 4–16 years of age with partial-onset seizures were assessed in double-blind, placebo-controlled safety profile studies. Levetiracetam has been shown to be no different (no less safe) from placebo in terms of changes from baseline on the Leiter-R Attention and Memory scale and the Memory Screen Composite scale.
in patients analyzed per protocol. The results of the study of behavioral and emotional functions, confirming that aggressive behavior occurs during the use of levetiracetam, were obtained using a standardized method using a validated instrument – the Achenbach Child Behavior Checklist.
However, in patients taking levetiracetam long-term in open-label studies, behavioral and emotional dysfunctions did not occur, in particular, the level of aggressive behavior did not differ from baseline.
Interaction
Interaction
Anticonvulsants
According to pre-marketing clinical studies, levetiracetam does not affect the serum concentrations of other anticonvulsants: phenytoin, carbamazepine, valproic acid, phenobarbital, lamotrigine, gabapentin and primidone, and these anticonvulsants do not affect the pharmacokinetics of levetiracetam.
However, there is evidence that the clearance of levetiracetam in children taking anticonvulsants that are inducers of microsomal liver enzymes increases by 22%.
Probenecid
Probenecid (500 mg 4 times daily) is a blocker of renal tubular secretion and has been shown to inhibit the renal clearance of the main metabolite, but not levetiracetam. However, the concentration of the main metabolite remains low.
It is expected that other drugs excreted by active tubular secretion may reduce the renal clearance of the drug.
metabolite. The effect of levetiracetam on probenecid has not been studied; The effect of levetiracetam on other drugs excreted by active tubular secretion, including non-steroidal anti-inflammatory drugs, sulfonamide and methotrexate, is not known.
Oral contraceptives and other pharmacokinetic interactions
Levetiracetam at a dose of 1000 mg per day did not affect the pharmacokinetics of oral contraceptives (ethinyl estradiol and levonorgestrel); hormonal status (the content of luteinizing hormone and progesterone) did not change.
Levetiracetam at a dose of 2000 mg per day had no effect on the pharmacokinetics of digoxin and warfarin, and the prothrombin time did not change. The simultaneous use of digoxin, oral contraceptives and warfarin did not affect the pharmacokinetics of levetiracetam.
Antacids
There are no data on the effect of antacids on the absorption of levetiracetam.
Food and alcohol
Food does not affect the extent of absorption of levetiracetam, but slightly reduces its rate. There are no data on the interaction of levetiracetam with ethanol.
Overdose
Overdose
Symptoms: drowsiness, agitation, aggressiveness, anxiety, depression of consciousness, respiratory depression, coma.
Treatment: in the acute period – artificial induction of vomiting and gastric lavage, followed by the administration of activated charcoal.
There is no specific antidote for levetiracetam. If necessary, symptomatic treatment is carried out in a hospital setting using hemodialysis (dialysis efficiency for levetiracetam is 60%, for its primary metabolite – 74%).
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C in the manufacturer’s packaging. Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Manufacturer
Manufacturer
Kanonpharma production CJSC, Russia
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25°C in the manufacturer's package. Keep out of reach of children. |
| Manufacturer | Kanonfarma Production ZAO, Russia |
| Medication form | pills |
| Brand | Kanonfarma Production ZAO |
Related products
Buy Levetiracetam Canon, 1000 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.