No products in the cart.
Lamotrigine Canon, tablets 100 mg 30 pcs
€21.93 €19.00
Description
Pharmacodynamics
Lamotrigine is a blocker of potential-dependent sodium channels. In neuronal culture it causes potential-dependent blockade of repetitive impulsation and suppresses pathological release of glutamic acid (an amino acid that plays a key role in the development of epileptic seizures), as well as inhibits glutamate-induced depolarization.
Pharmacokinetics
Absorption
Lamotrigine is rapidly and completely absorbed from the intestine with little or no presystemic first-pass metabolism. The maximum concentration (Cmax) in blood plasma is reached approximately 2.5 hours after oral administration of the drug. Time of reaching Cmax is slightly increased after meals, but the degree of absorption remains unchanged. Pharmacokinetics is linear with a single dose up to 450 mg (the highest dose studied).
Distribution
Lamotrigine binds to plasma proteins by approximately 55%. The volume of distribution (Vp) is 0.92-1.22 l/kg.
Metabolism
The enzyme uridine diphosphate glucuronyltransferase (UDF-glucuronyltransferase) is involved in lamotrigine metabolism. Lamotrigine increases its own metabolism to a small extent in a dose-dependent manner.
Elimination
In healthy adults, the clearance of lamotrigine at equilibrium concentrations averages 39 ± 14 mL/min. Lamotrigine is metabolized to glucuronides, which are excreted by the kidneys. Less than 10% of the drug is excreted unchanged by the kidneys, about 2% – by the intestine. Clearance and half-life (T½) do not depend on the dose. T½ in healthy adults averages 24 h to 35 h. In patients with Gilbert’s syndrome a 32 % decrease in drug clearance was observed compared to the control group, however, this was within the normal range for the general population. The average half-life is reduced to approximately 14 h when concomitantly administered with glucuronizing drugs such as carbamazepine and phenytoin, and increased to an average of 70 h when concomitantly administered with valproate.
In children, lamotrigine clearance per body weight is higher than in adults; it is highest in children under 5 years of age. T½ is usually shorter than in adults. It averages approximately 7 h when co-administered with glucuronizing drugs such as carbamazepine and phenytoin, and increases to an average of 45 to 50 h when co-administered with valproate.
In elderly patients, clinically significant differences in lamotrigine clearance compared to younger patients have not been found.
If renal function is significantly impaired, a dose reduction of lamotrigine may be required.
In patients with moderate to severe hepatic impairment, doses of lamotrigine should be reduced.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
One tablet contains:
Active ingredient:
Associates:
Hyprolose (hydroxypropyl cellulose) 11 mg,
sodium carboxymethyl starch 9.5 mg,
lactose monohydrate 120 mg,
magnesium hydroxycarbonate 77 mg,
magnesium stearate 2.5 mg
How to take, the dosage
How to take, the dosage
Ingestion. If the calculated dose of lamotrigine (e.g., when administered to children or patients with impaired liver function) cannot be divided into a whole number of lower-dose tablets, the patient should be prescribed a dose that corresponds to the nearest value of the whole lower-dose tablet.
In patients taking medications whose pharmacokinetic interaction with lamotrigine is currently unknown, the regimen recommended for prescribing lamotrigine in combination with valproic acid preparations should be used. In children with a body weight less than 25 kg or if the calculated maintenance dose in children is less than 25 mg/day, Lamotrigine Canon should not be administered.
If lamotrigine is restarted, physicians should evaluate the need to increase the dose in patients who have discontinued the drug for any reason, since high starting doses and exceeding recommended doses are associated with a risk of developing a severe rash. The longer the time since the last drug administration, the more caution should be exercised to increase the dose to maintenance. If the time since discontinuation exceeds 5 half-lives, the lamotrigine dose should be increased to maintenance according to an appropriate regimen.
It is not recommended that lamotrigine be restarted in patients who have discontinued the drug due to a rash unless the potential benefits of the drug clearly outweigh the possible risks.
Interaction
Interaction
UDF-glucuronyltransferase is the main enzyme that metabolizes lamotrigine. There are no data on the ability of lamotrigine to cause clinically significant induction or inhibition of microsomal liver enzymes.
In this regard, interactions between lamotrigine and drugs metabolized by cytochrome P450 isoenzymes are unlikely. Lamotrigine may induce its own metabolism, but this effect is moderate and has no clinically significant consequences.
Special Instructions
Special Instructions
Skin rash
There have been reports of adverse skin reactions that may occur within the first 8 weeks of starting lamotrigine therapy. Most rashes are mild and resolve on their own, but there are reports of rashes that have required hospitalization and discontinuation of lamotrigine.
These have included potentially life-threatening skin reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis (Lyell syndrome). Severe skin reactions in adult patients using lamotrigine according to generally accepted guidelines develop at a rate of about 1 in 500 patients with epilepsy.
In about half of these cases, Stevens-Johnson syndrome is reported (1 in 1,000). In patients with bipolar disorders, the incidence of severe skin rashes according to clinical studies is approximately 1 per 1,000 patients. Children have a higher risk of developing severe skin rashes than adults. In children, an initial rash may be mistaken for an infection, so physicians should consider the possibility of children reacting to the drug by developing a rash and fever during the first 8 weeks of therapy.
Caution is necessary when prescribing to patients with a history of allergic reactions or rash in response to other antiepileptic drugs because the incidence of rash (not classified as severe) in patients with such a history was observed three times as often when prescribed lamotrigine than in patients with an unweighted history. All patients should be seen by a physician immediately if a rash is detected.
Lamotrigine administration should be discontinued immediately unless it is clear that the development of the rash is not related to taking the drug. It is not recommended to resume lamotrigine administration in cases where its previous prescription has been discontinued due to the development of a skin reaction, unless the expected therapeutic effect of the drug exceeds the risk of side effects. It has been reported that rash may be part of a hypersensitivity syndrome associated with various systemic manifestations, including fever, lymphadenopathy, facial swelling, and blood and liver disorders.
The severity of the syndrome varies widely and in rare cases may lead to the development of disseminated intravascular coagulation (DIC) syndrome and multiple organ failure. It should be noted that early manifestations of hypersensitivity syndrome (fever, lymphadenopathy) may be observed even if there are no obvious manifestations of rash. If such symptoms develop, the patient should be immediately examined by a physician and, if no other cause of the symptoms is established, lamotrigine should be withdrawn.
The risk of aseptic meningitis
It has been reported that children and adults taking lamotrigine have an increased risk of aseptic meningitis. Patients should be advised to see a physician immediately if they develop symptoms and signs of meningitis. If meningitis develops, the physician should discontinue therapy with lamotrigine. When the drug is withdrawn, in most cases the symptoms of meningitis disappeared, but in some patients they recurred when lamotrigine was re-prescribed. Patients who have discontinued the drug due to aseptic meningitis associated with prior lamotrigine administration should not be restarted.
Clinical deterioration and suicidal risk
Suicidal ideation and suicidal behavior have been reported in patients taking PEP for several indications, including epilepsy and bipolar disorder. The mechanism of this action is unknown, and the available data do not rule out the possibility of an increased risk of suicide with lamotrigine. Thus, patients taking lamotrigine should be closely monitored for suicidal ideation and behavior. Patients (and caregivers) should be informed of the need for medical consultation if such symptoms occur.
Dehydrofolate reductase
Lamotrigine is a weak inhibitor of dehydrofolate reductase, so there is potential for the drug to interfere with folate metabolism during long-term therapy. However, lamotrigine has not been shown to cause significant changes in hemoglobin concentration, mean erythrocyte volume, and serum folate concentration when prescribed for up to 1 year and did not decrease erythrocyte folate concentration when lamotrigine was prescribed for up to 5 years.
Renal failure
Single administration of lamotrigine to patients in end-stage renal failure showed no significant changes in drug concentrations. However, accumulation of the glucuronide metabolite is very likely, so caution should be exercised when treating patients with renal insufficiency.
Patients taking other drugs containing lamotrigine
Do not prescribe Lamotrigine Kanon to patients already receiving other drugs containing lamotrigine without medical advice.
Epilepsy.
Abrupt withdrawal of lamotrigine, as well as other PEPs, may provoke the development of seizures. If abrupt discontinuation of therapy is not a safety requirement (e.g., if a rash occurs), the lamotrigine dose should be reduced gradually over 2 weeks. There are reports that severe seizures, including status epilepticus, may lead to the development of rhabdomyolysis, multiple organ disorders and disseminated intravascular coagulation, sometimes with fatal outcome. Similar cases have been observed in patients treated with lamotrigine.
Bipolar affective disorder
Patients with bipolar affective disorder may experience clinical deterioration and/or worsening of suicidal ideation and suicidal behavior when taking bipolar disorder medications, including lamotrigine. Patients taking lamotrigine should be closely monitored during treatment. Patients (and caregivers) should be informed to monitor for any deterioration in patients’ condition, including the appearance of new symptoms and/or suicidal thoughts/behavior, and to seek medical attention immediately if these symptoms are present. In doing so, the situation should be evaluated and appropriate changes made to the therapy regimen, including the possibility of withdrawing the drug.
When using Lamotrigine Canon, it is recommended to refrain from driving vehicles and activities requiring increased concentration and speed of psychomotor reactions.
Contraindications
Contraindications
With caution
Disorders of liver and kidney function.
Side effects
Side effects
Epilepsy
Skin and subcutaneous tissue
Very common: skin rash.
Rare: Stevens-Johnson syndrome.
Very rare: toxic epidermal necrolysis.
Blood and lymphatic system disorders
Very rare: neutropenia, leukopenia, anemia, thrombocytopenia, pancytopenia, aplastic anemia, agranulocytosis, lymphadenopathy.
In immune system disorders
very rarely: hypersensitivity syndrome (including such symptoms as fever, lymphadenopathy, facial edema, blood and liver function disorders, disseminated intravascular coagulation (DIC) syndrome, multiple organ failure).
Psychiatric disorders
Often: aggressiveness and irritability.
Very rarely: tics, hallucinations and mental confusion.
Nervous system disorders
In monotherapy
Very common: headache; common: drowsiness, insomnia, dizziness, tremor; infrequent: ataxia; rare: nystagmus.
In combination therapy
Very common: somnolence, ataxia, headache, dizziness; common: nystagmus, tremor, insomnia.
Rare: aseptic meningitis.
Very rare: agitation, unsteady gait, motor disorders, worsening of Parkinson’s disease symptoms, extrapyramidal disorders, choreoathetosis, increased frequency of seizures.
Visual disorders
In monotherapy
Infrequent: diplopia, blurred vision.
In combination therapy
Very common: diplopia, blurred vision; rarely: conjunctivitis.
Gastrointestinal tract
In monotherapy
Often: nausea, vomiting, diarrhea.
In combination therapy
Very common: nausea, vomiting.
Often: diarrhea.
Hepatic and biliary tract disorders
Very rare: increased activity of “liver” enzymes, liver dysfunction, liver failure. Liver function abnormalities usually develop in conjunction with symptoms of hypersensitivity, but in isolated cases have also been reported in the absence of obvious signs of hypersensitivity.
Skeletal, muscular and connective tissue disorders
Very rare: lupus-like syndrome; frequency unknown: osteomalacia, osteoporosis, bone fractures (especially in patients taking lamotrigine for a long time, when combined with other PEDs).
General disorders
Frequently: fatigue.
Bipolar affective disorder.
Skin and subcutaneous tissue
Very common: skin rash.
Rare: Stevens-Johnson syndrome.
In an evaluation of all studies (controlled and uncontrolled) of lamotrigine in patients with bipolar affective disorder, skin rash occurred in 12% of all patients receiving lamotrigine, whereas the incidence of skin rash in controlled studies alone was 8% in patients receiving lamotrigine and 6% in patients receiving placebo.
Nervous system disorders
Very common: headache.
Often: agitation, drowsiness, dizziness.
Gastrointestinal system
Often: dryness of the oral mucosa.
Musculoskeletal and connective tissue disorders
Often: arthralgia.
General disorders
Often: pain, back pain.
Overdose
Overdose
Symptoms: single administration of doses exceeding the maximum therapeutic by 10-20 times has been reported, including cases with fatal outcome. Overdose has manifested with symptoms including nystagmus, ataxia, impaired consciousness and coma, and possible enlargement of the QRS complex on the ECG.
Treatment: hospitalization and supportive therapy according to clinical presentation or national poison center guidelines are recommended.
Similarities
Similarities
Additional information
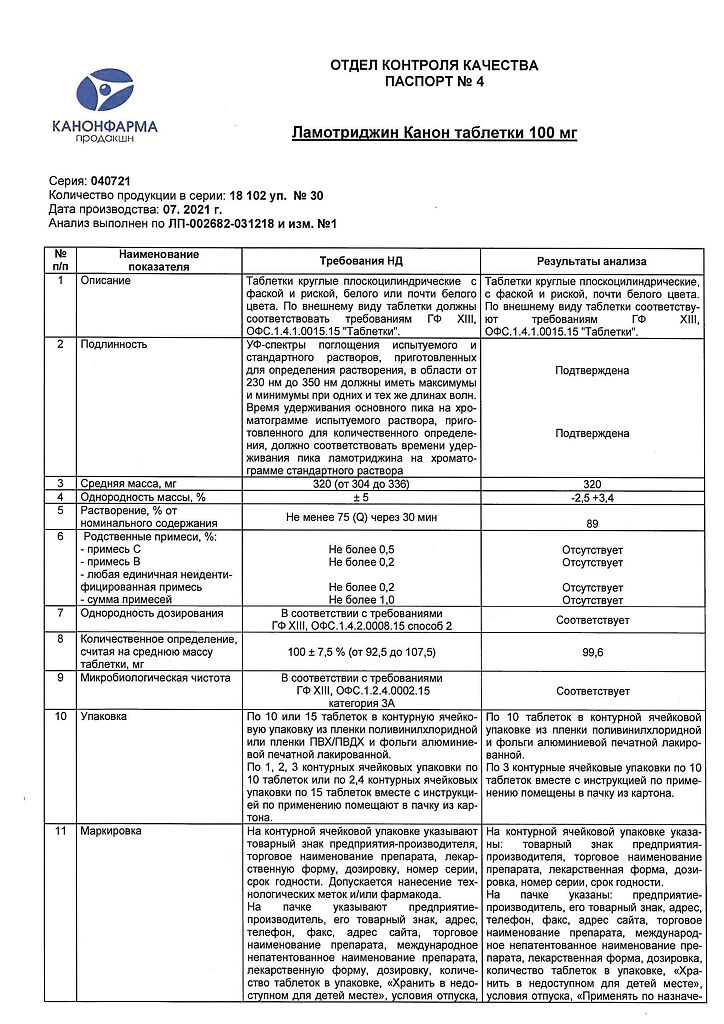
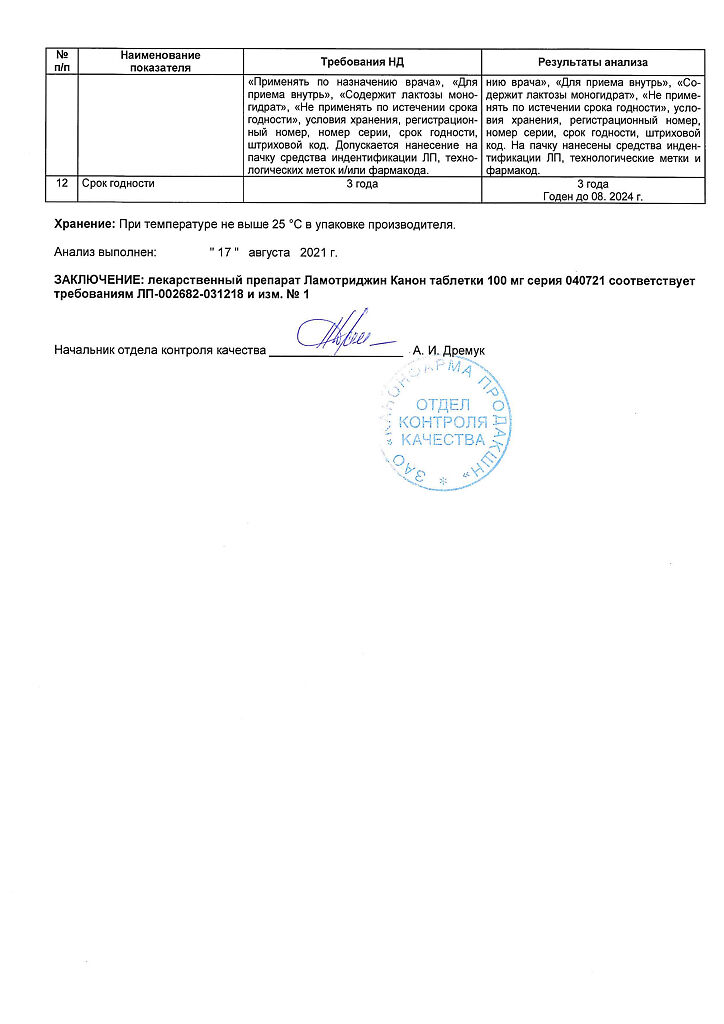
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature no higher than 25 ° C. |
| Manufacturer | Kanonfarma Production ZAO, Russia |
| Medication form | pills |
| Brand | Kanonfarma Production ZAO |
Related products
Buy Lamotrigine Canon, tablets 100 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.