No products in the cart.
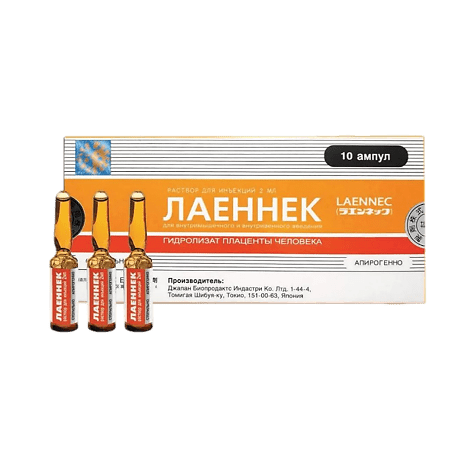
Laennec, 2 ml 50 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Laennek is an immunomodulatory and hepatoprotective drug.
The drug exhibits immunomodulatory properties due to the ability to stimulate humoral immunity and increase the functional activity of phagocytes and natural killer cells.
It increases bactericidal activity of leukocytes of peripheral blood that shows their ability to destroy invaded pathogen. Cytokines in the drug activate the metabolic and surveillance functions of skin cells.
The biologically active substances contained in the hydrolyzate stimulate the regeneration (proliferation) of hepatocytes, exhibit detoxifying properties, reduce deposition of lipids and cholesterol in liver cells, increase the activity of tissue respiration, activate metabolism in the liver, reduce the intensity of connective tissue in the liver.
Indications
Indications
Active ingredient
Active ingredient
How to take, the dosage
How to take, the dosage
In chronic recurrent herpes and atopic dermatitis the drug is administered by IV drop: 10 ml (560 mg of placenta hydrolysate) of the drug (5 ampoules) are dissolved in 250-500 ml of 5% dextrose solution or physiological solution and injected through the ulnar vein for 1.5-2 hours. Injections are given 3 times a week at 2-day intervals. The course of treatment is 10 injections.
In chronic liver diseases (steatohepatitis /alcoholic, metabolic and mixed etiology/) the drug is administered in/m 2 ml/day (112 mg of placenta hydrolysate). Depending on the severity of the disease, frequency of injections can be increased up to 2-3 times (6 ml)/day. The drug can be administered by IV drop: 10 ml (560 mg of placenta hydrolysate) of the drug (5 ampoules) are dissolved in 250-500 ml of 5% dextrose solution or physiological solution and injected through the ulnar vein for 1.5-2 hours. Injections are carried out twice a week. The course of treatment is 2-3 weeks.
Interaction
Interaction
The activity of Laennec solution when mixed with other drugs that are strong bases (pH above 8.5) decreases.
To date no other clinically significant drug interactions have been identified.
Special Instructions
Special Instructions
According to currently available data, the drug can be administered to the elderly. However, given that physiological functions worsen in the elderly, the drug should be used under close supervision.
Paediatric use
Studies on the safety of Laennec in infants (including premature infants) and juveniles have not been conducted. Use in children is not recommended.
Contraindications
Contraindications
Patients with multivalent drug allergy and the elderly should be used with caution.
Side effects
Side effects
Side effects have been reported in 3.7% of patients.
Clinically significant adverse reactions: allergic reactions possible.
Other adverse events: soreness at the injection site (2.56%), allergic reactions (redness, itching) (0.37%), numbness at the injection site (0.37%), gynecomastia (0.37%) – no causal relationship to the drug administration was found.
Overdose
Overdose
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | The drug should be stored in the dark place out of the reach of children at 18 ° to 25 ° C. |
| Manufacturer | Japan Bio Products Co. |
| Medication form | solution for injection |
| Brand | #Н/Д |
Related products
Buy Laennec, 2 ml 50 pcs with delivery to USA, UK, Europe and over 120 other countries.