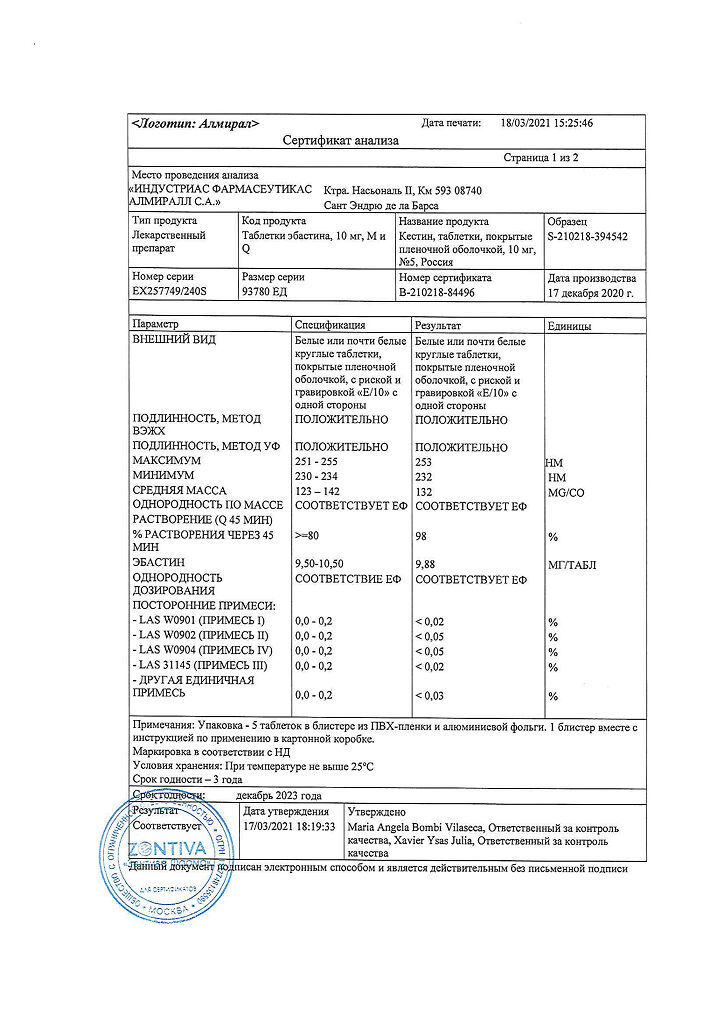
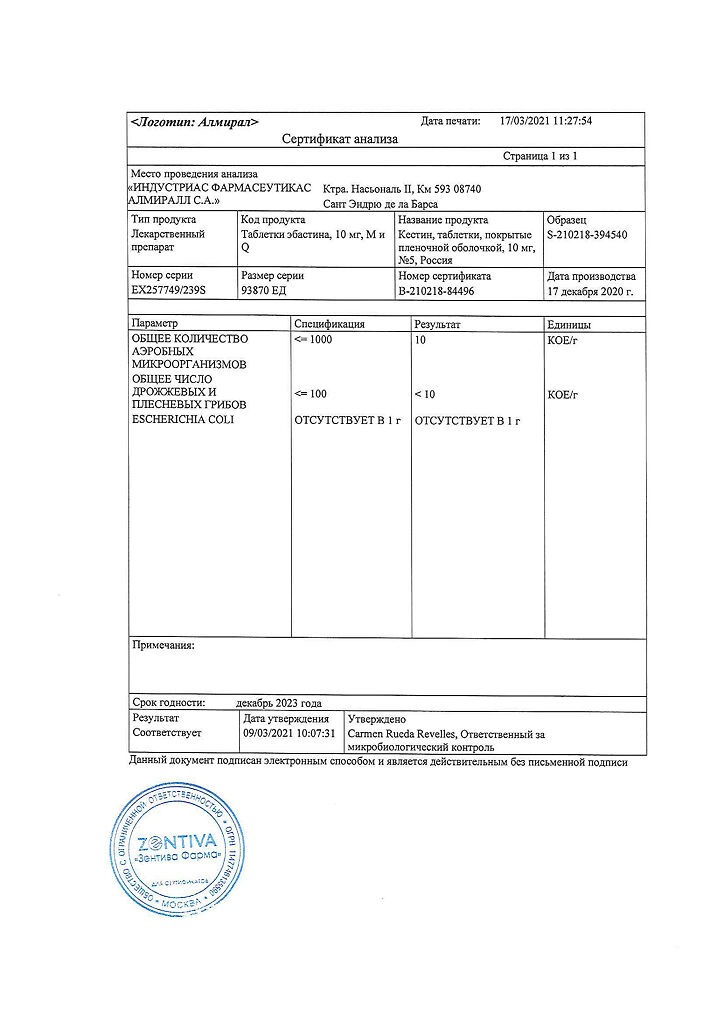
No products in the cart.
Description
Pharmacodynamics
Anti-allergic drug. After oral administration a pronounced antiallergic effect starts after 1 hour and lasts for 48 hours. After 5-day course of treatment with Kestin® antihistamine activity is maintained for 72 hours due to the action of active metabolites. The drug has no pronounced anticholinergic and sedative effect. No effect of Kestin® on ECG QT-interval was observed at a dose of 100 mg that was 5-10 times higher than the recommended daily dose.
Pharmacokinetics
After oral administration, the drug is rapidly absorbed and almost completely metabolized in the liver, converting to the active metabolite carabastine. After a single dose of 10 mg of the drug, the Cmax of carabastine in plasma is reached after 2.6-4 hours and is 80-100 ng/ml. It does not penetrate the blood-brain barrier.
When taking 10 mg of the drug daily, the equilibrium concentration is reached after 3-5 days and is 130-160 ng/ml. Binding to plasma proteins of ebastine and carabastine is more than 95%. T1/2 of carabastine is from 15 to 19 hours, 66% of the drug is excreted as conjugates through the kidneys.
Eating has no effect on the clinical effects of the drug Kestin®.
In elderly patients the pharmacokinetic parameters do not change significantly. In renal insufficiency the T1/2 is increased up to 23-26 h and in hepatic insufficiency – up to 27 h, but the drug concentration does not exceed therapeutic values.
Indications
Indications
Allergic rhinitis of various etiologies (seasonal and/or year-round, both accompanied and not accompanied by allergic conjunctivitis).
Urticaria of various etiologies, including chronic idiopathic.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Antiallergic drug. After taking the drug orally, a pronounced antiallergic effect begins within 1 hour and lasts for 48 hours. After a 5-day course of treatment with Kestin®, antihistamine activity persists for 72 hours due to the action of active metabolites. The drug does not have a pronounced anticholinergic and sedative effect. There was no effect of the drug Kestin® on the QT interval of the ECG at a dose of 100 mg, which exceeds the recommended daily dose by 5-10 times.
Pharmacokinetics
After oral administration, it is quickly absorbed and almost completely metabolized in the liver, turning into the active metabolite carabastine. After a single dose of 10 mg of the drug, Cmax of carabastine in plasma is reached after 2.6-4 hours and is 80-100 ng/ml. Does not penetrate the blood-brain barrier.
When taking 10 mg of the drug daily, the equilibrium concentration is reached after 3-5 days and is 130-160 ng/ml. Plasma protein binding of ebastine and carabastine is more than 95%. T1/2 of carabastine ranges from 15 to 19 hours, 66% of the drug is excreted in the form of conjugates through the kidneys.
Food intake does not affect the clinical effects of Kestin®.
In elderly patients, pharmacokinetic parameters do not change significantly. In case of renal failure, T1/2 increases to 23-26 hours, and in case of liver failure – up to 27 hours, however, the concentration of the drug does not exceed therapeutic values.
Special instructions
Special instructions
Impact on the ability to drive vehicles and other mechanisms that require increased concentration of attention
In the event of side effects from the central nervous system, there may be a minimal decrease in the ability of patients to drive vehicles and engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Ebastine
Composition
Composition
Active ingredient:
ebastine microionized 10 mg.
Excipients:
microcrystalline cellulose,
corn starch,
lactose monohydrate,
sodium carboxymethylcellulose structured,
magnesium stearate,
hydroxypropyl methylcellulose,
polyethylene glycol 6000,
titanium dioxide
Pregnancy
Pregnancy
Contraindicated during pregnancy and lactation.
Contraindications
Contraindications
Hypersensitivity to the components of the drug;
pregnancy and lactation;
children under 12 years of age;
lactase deficiency, lactose intolerance, glucose-galactose malabsorption.
With caution: use in patients with an increased QT interval, hypokalemia, renal and/or liver failure.
Side Effects
Side Effects
With a frequency of more than 1%: headache (7.9%), drowsiness (3.0%), dry oral mucosa (2.1%).
With a frequency of less than 1%: dyspepsia, nausea, insomnia, abdominal pain, asthenic syndrome, sinusitis, rhinitis.
Interaction
Interaction
It is not recommended to use Kestin® simultaneously with ketoconazole and erythromycin (increased risk of QT interval prolongation).
The drug Kestin® does not interact with theophylline, indirect anticoagulants, cimetidine, diazepam, ethanol and ethanol-containing drugs.
Overdose
Overdose
Symptoms: signs of moderate effects on the central nervous system (fatigue) and autonomic nervous system (dry oral mucosa) can only occur with high doses (300 mg-500 mg, which is 30-50 times higher than the therapeutic dose).
Treatment: There is no special antidote for ebastine. In case of overdose, gastric lavage, monitoring of vital body functions, and symptomatic treatment are recommended.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 30 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Industrias Pharmaceuticas Almiralle S.A., Spain
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 30 °C |
| Manufacturer | Almiral S.A., Spain |
| Medication form | pills |
| Brand | Almiral S.A. |
Other forms…
Related products
Buy Kestin, 10 mg 5 pcs. with delivery to USA, UK, Europe and over 120 other countries.