No products in the cart.
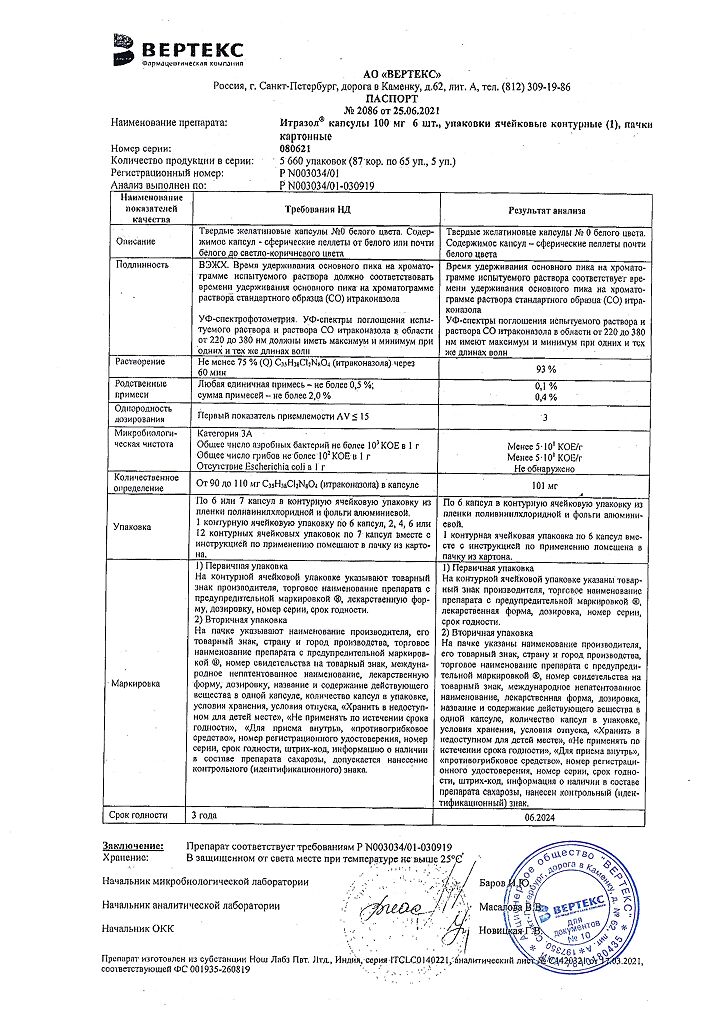
Itrazol, 100 mg capsules 6 pcs
€14.07 €12.20
Description
Itraconazole is an antifungal.
Pharmacodynamics
Itraconazole is a synthetic broad-spectrum antifungal agent, a triazole derivative. It inhibits the synthesis of the cell membrane ergosterol of fungi, which determines the antifungal effect of the drug.
Itraconazole is active against infections caused by dermatophytes (Trichophyton spp.,Microsporum spp., Epidermophyton floccosum), yeast-like fungi and yeasts (Cryptococcus neoformans, Pityrosporum spp., Candida spp. including C albicans, C. glabrata, and C. krusei); Aspergillus spp., Histoplasma spp., Paracoccidioides brasiliensis, Sporothrix schenckii, Fonsecaea spp., Cladosporium spp., Blastomyces dermatidis, and other yeasts and molds.
Pharmacokinetics
It is absorbed from the gastrointestinal tract quite completely. Taking itraconazole in capsules immediately after a meal increases bioavailability. Cmax in plasma is reached within 3-4 hours after oral administration. Css when taking 100 mg of the drug once a day is 0.4 mcg/ml; when taking 200 mg once a day – 1.1 mcg/ml, 200 mg 2 times a day – 2 mcg/ml.
The time of onset of Css in plasma with prolonged use is 1-2 weeks. The binding to plasma proteins is 99.8%.
It penetrates well into tissues and organs (including vaginal mucosa), is contained in the secretion of sebaceous and sweat glands. Concentration of itraconazole in lungs, kidneys, liver, bones, stomach, spleen, skeletal muscles is 2-3 times higher than its concentration in plasma; in tissues containing keratin – 4 times higher.
Therapeutic concentration of itraconazole in skin is maintained for 2-4 weeks after discontinuation of 4-week course of treatment. The therapeutic concentration in the keratin of the nails is reached 1 week after the start of treatment and is maintained for 6 months after the completion of the 3-month course of treatment. Low concentrations are determined in the sebaceous and sweat glands of the skin.
It is metabolized in the liver with the formation of active metabolites, including hydroxyitraconazole. It is an inhibitor of CYP3A4, CYP3A5 and CYP3A7 isoenzymes.
Indications
Indications
ringworm;
fungal keratitis;
onychomycosis caused by dermatophytes and/or yeasts and molds;
systemic mycoses: systemic aspergillosis and candidiasis, cryptococcosis (including cryptococcal meningitis), histoplasmosis, sporotrichosis, paracoccidioidomycosis, blastomycosis and other systemic or tropical mycoses;
candidomycosis with damage to the skin or mucous membranes, incl. vulvovaginal candidiasis;
pityriasis versicolor.
Pharmacological effect
Pharmacological effect
Intrazol is an antifungal.
Pharmacodynamics
Itraconazole is a synthetic broad-spectrum antifungal agent, a triazole derivative. Inhibits the synthesis of ergosterol in the cell membrane of fungi, which determines the antifungal effect of the drug.
Itraconazole is active against infections caused by dermatophytes (Trichophyton spp., Microsporum spp., Epidermophyton floccosum), yeast-like fungi and yeasts (Cryptococcus neoformans, Pityrosporum spp., Candida spp., including C. albicans, C. glabrata and C. krusei); Aspergillus spp., Histoplasma spp., Paracoccidioides brasiliensis, Sporothrix schenckii, Fonsecaea spp., Cladosporium spp., Blastomyces dermatidis, as well as other yeasts and molds.
Pharmacokinetics
Absorbed from the gastrointestinal tract quite completely. Taking itraconazole capsules immediately after meals increases bioavailability. Cmax in plasma is achieved within 3–4 hours after oral administration. Css when taking 100 mg of the drug once a day – 0.4 mcg/ml; when taking 200 mg 1 time per day – 1.1 mcg/ml, 200 mg 2 times a day – 2 mcg/ml.
The time for the onset of Css in plasma with long-term use is 1–2 weeks. Bonding with plasma proteins is 99.8%.
Penetrates well into tissues and organs (including the vaginal mucosa), and is contained in the secretion of the sebaceous and sweat glands. The concentration of itraconazole in the lungs, kidneys, liver, bones, stomach, spleen, and skeletal muscles is 2–3 times higher than its concentration in plasma; in tissues containing keratin – 4 times.
The therapeutic concentration of itraconazole in the skin persists for 2–4 weeks after cessation of the 4-week course of treatment. The therapeutic concentration in nail keratin is achieved 1 week after the start of treatment and remains for 6 months after completion of the 3-month course of treatment. Low concentrations are detected in the sebaceous and sweat glands of the skin.
Metabolized in the liver to form active metabolites, incl. hydroxyitraconazole. It is an inhibitor of the isoenzymes CYP3A4, CYP3A5 and CYP3A7.
Excretion from plasma is two-phase: by the kidneys within 1 week (35% in the form of metabolites, 0.03% in unchanged form) and through the intestines (3–18% in unchanged form). T1/2 – 1–1.5 days. It is not removed during dialysis.
Special instructions
Special instructions
The treatment regimen and dosage are prescribed by the doctor.
For optimal absorption of the drug, it is necessary to take ITRAZOL® capsules immediately after meals.
Women of childbearing potential taking Itrazol® must use adequate contraception throughout the course of treatment until the first menstrual period after its completion.
When studying the IV dosage form of itraconazole, a transient asymptomatic decrease in left ventricular ejection fraction was noted, which normalized until the next infusion of the drug.
Itraconazole has a negative inotropic effect. Cases of heart failure associated with itraconazole have been reported. Itraconazole should not be taken by patients with CHF or a history of this disease unless the possible benefit significantly outweighs the potential risk.
CCBs may have a negative inotropic effect, which may enhance this effect of itraconazole; itraconazole may reduce the metabolism of CCBs. Caution should be exercised when taking itraconazole and CCBs concomitantly.
With reduced gastric acidity, the absorption of itraconazole is impaired. For patients taking antacid medications (for example, aluminum hydroxide), it is recommended that they be used no earlier than 2 hours after taking Itrazol® capsules. Patients with achlorhydria or using H2-histamine blockers or proton pump inhibitors are recommended to take Itrazole® capsules with acidic drinks.
With long-term use of itraconazole (more than 1 month), when using itraconazole in patients receiving other drugs that have hepatotoxic effects, as well as in patients with liver diseases, it is recommended to regularly monitor liver function. Patients should be advised to contact their physician immediately if symptoms suggestive of hepatitis occur, such as anorexia, nausea, vomiting, weakness, abdominal pain, and dark urine. If such symptoms occur, you should immediately stop therapy and conduct a liver function test.
In patients with renal failure, the bioavailability of itraconazole may be reduced, and therefore dose adjustment is necessary.
Treatment should be discontinued if neuropathy occurs, which may be associated with taking Itrazole® capsules. There is no evidence of cross-hypersensitivity to itraconazole and other azole antifungals. Itrazole® capsules should be administered with caution to patients with hypersensitivity to other azoles.
In patients with compromised immunity (AIDS, after organ transplantation, neutropenia), an increase in the dose of Itrazole® may be required.
Active ingredient
Active ingredient
Itraconazole
Composition
Composition
1 capsule contains:
Active substance:
itraconazole 100 mg;
Excipients:
sugar pellets (sucrose – 80-91.5%, corn starch – 8.5-20%) – 207.44 mg;
poloxamer 188 (Lutrol) – 25.94 mg;
Poloxamer 188 (Lutrol) micronized – 0.51 mg;
hypromellose – 130.11 mg.
Pregnancy
Pregnancy
During pregnancy and breastfeeding, Itrazol is prescribed only in cases where the expected effect of treatment outweighs the possible risk of side effects.
Contraindications
Contraindications
individual hypersensitivity to the drug or its components;
simultaneous use of drugs metabolized by the CYP3A4 enzyme: terfenadine, astemizole, mizolastine, cisapride, dofetilide, quinidine, pimozide, HMG-CoA reductase inhibitors such as simvastatin and lovastatin, triazolam and midazolam (see “Interaction”);
pregnancy;
lactation period;
children’s age (up to 3 years).
With caution: severe heart failure; liver diseases (including those accompanied by liver failure). It is recommended to use Itrazol® in children over 3 years of age only if the possible benefit outweighs the potential risk.
Side Effects
Side Effects
From the gastrointestinal tract: dyspepsia, nausea, abdominal pain and constipation, reversible increase in the activity of liver enzymes, cholestatic jaundice, hepatitis, anorexia. In very rare cases, when using the drug Itrazol®, severe toxic damage to the liver developed, incl. a case of acute liver failure with a fatal outcome.
From the central nervous system: headache, fatigue, dizziness, peripheral neuropathy.
From the cardiovascular system: congestive heart failure and pulmonary edema.
From other organs and systems: menstrual irregularities, allergic reactions (such as itching, rash, urticaria and angioedema), Stevens-Johnson syndrome, alopecia, hypokalemia, edema, dark urine discoloration, hypercreatininemia.
Interaction
Interaction
Drugs that affect the metabolism of itraconazole
The interaction of itraconazole with rifampicin, rifabutin and phenytoin has been studied. The simultaneous use of itraconazole with these drugs, which are potential inducers of liver enzymes, is not recommended. Interaction studies with other hepatic enzyme inducers such as carbamazepine, phenobarbital and isoniazid have not been conducted, but similar results can be expected due to the fact that itraconazole is primarily metabolized by the enzyme CYP3A4; potent inhibitors of this enzyme may increase the bioavailability of itraconazole. Examples include ritonavir, indinavir, clarithromycin and erythromycin.
The effect of itraconazole on the metabolism of other drugs
Itraconazole may inhibit the metabolism of drugs metabolized by the CYP3A4 enzyme. The result of this may be an intensification or prolongation of their action, incl. and side effects.
Drugs that should not be given concomitantly with itraconazole
– terfenadine, astemizole, mizolastine, cisapride, triazolam and oral midazolam, dofetilide, quinidine, pimozide, HMG-CoA reductase inhibitors such as simvastatin and lovastatin;
– CCBs may have a negative inotropic effect, which may enhance the same effect exhibited by itraconazole. When taking itraconazole and CCBs simultaneously, caution must be exercised because CCB metabolism may be reduced.
Drugs, when prescribed, it is necessary to monitor their concentration in plasma and their effect, side effects
When co-administered with itraconazole, the dose of the following drugs should be reduced, if necessary:
– oral anticoagulants;
– HIV protease inhibitors, such as ritonavir, indinavir, saquinavir;
– some antitumor drugs, such as rose vinca alkaloids, busulfan, docetaxel, trimetrexate;
– CCBs cleaved by the CYP3A4 enzyme, such as verapamil;
– some immunosuppressive drugs: cyclosporine, tacrolimus, sirolimus;
– other drugs: digoxin, carbamazepine, buspirone, alfentanil, alprazolam, brotizolam, rifabutin, methylprednisolone, ebastine, reboxetine.
No interaction was found between itraconazole and zidovudine and fluvastatin.
There was no effect of itraconazole on the metabolism of ethinyl estradiol and norethisterone.
Effect on protein binding
In vitro studies have demonstrated the lack of competition between itraconazole and drugs such as imipramine, propranolol, diazepam, cimetidine, indomethacin, tolbutamide and sulfadimidine in binding to plasma proteins.
Overdose
Overdose
No data available.
Treatment: during the first hour, perform gastric lavage and, if necessary, prescribe activated charcoal and symptomatic treatment. Itraconazole is not eliminated by hemodialysis. There is no specific antidote for the drug.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
2.5 years
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 2.5 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Vertex, Russia |
| Medication form | capsules |
| Brand | Vertex |
Other forms…
Related products
Buy Itrazol, 100 mg capsules 6 pcs with delivery to USA, UK, Europe and over 120 other countries.